Meeting Summary
Prof Peter van de Kerkhof, Radboud University Nijmegen Medical Centre, the Netherlands, discussed the very long history, particularly in Germany and the Netherlands, of dimethyl fumarate (a fumaric acid ester [DMF]) in treating psoriasis in adults. However, only recently, with a new DMF product registration in Europe, has clinical research escalated to investigate the most effective clinical use of this agent. Treatment strategies in patients with psoriasis should be personalised, with considerations of the disease, patient characteristics and preferences, as well as current and historical treatments. He presented a case scenario to highlight which patients might benefit most from DMF as a first-line systemic treatment for moderate-to-severe psoriasis. He further discussed the role of acitretin (a synthesised retinoic acid) as monotherapy in pustular psoriasis and erythrodermic psoriasis, and in combination for chronic plaque psoriasis. He concluded that in patients requiring systemic therapy with contraindications for immunosuppressive therapies, DMF and acitretin may provide a unique profile and treatment solution.
Fumarates and Vitamin A Derivatives
Professor Peter van de Kerkhof
The fumarates, monoethyl fumarate (MEF) and dimethyl fumarate (DMF), are potent ester derivatives of fumaric acid. Fumaric acid esters (FAE) are indicated for and have been used for >25 years for the treatment of moderate-to-severe plaque psoriasis in adults in need of systemic medicinal therapy.1 In fact, FAE (available in a fixed combination) have become the most frequently used first-line systemic therapy in Germany and the Netherlands, with increasing use in north-western Europe.2,3 Recent registration for Skilarence® (Almirall SA., Spain), a product containing only DMF, with the European Medicines Agency (EMA),1,3 has intensified regulated long-term pharmacovigilance and active clinical research to determine the proper clinical use, effectiveness, and safety of DMF in the treatment of psoriasis and other skin conditions.4
FAE are recommended in the European S3-Guidelines on the systemic treatment of psoriasis vulgaris for induction and long-term treatment of adults with moderate-to-severe chronic plaque psoriasis. This decision was based on high-quality clinical evidence and expert consensus. Furthermore, these international guidelines recommend applying a slow, gradual step-by-step dose increasing regimen to minimise gastrointestinal side effects, such as nausea and abdominal pain.3,5,6
Treatment strategies in patients with psoriasis should be personalised with considerations of the severity of skin signs and symptoms: disease burden; patient characteristics, such as age, gender, needs, and preferences; associated diseases; current treatments; response to, and/or intolerability of, historical treatments; and the availability of products.7
The advancement in treatment options like DMF highlights the importance of personalised treatment selection. For example, if a patient has an unstable form of psoriasis, their disease can rapidly progress to a serious medical complication, so systemic therapy may be indicated.8 In addition, it is critical to identify and address any modifiable triggers and exacerbating factors, such as infections.5,8 If a patient has a history of cardiovascular disease, it is important to assess and monitor cardiovascular disease risk to reduce the comorbid risk9 and to strive for optimal and early systemic treatment.5 Treatment and care should consider patients’ needs and preferences,9 so if a patient feels that there is no urgent need for a rapid resolution and is happy with a gradual but definite improvement without the use of topicals, DMF is an appropriate option. If a patient has a concomitant disease, such as chronic asthmatic bronchitis requiring occasional antibiotics, or if a patient is exposed to areas of endemic tuberculosis, immunosuppressive therapy may be contraindicated.5
Prof van de Kerkhof presented the following case study to illustrate the positioning of DMF and the importance of personalised treatment. As the patient demonstrates unstable psoriasis, which is an indication for requiring systemic therapy, Prof van de Kerkhof outlined that DMF would be his first-line choice and acitretin would be the second choice. The reason for selecting DMF is that the patient requested a gradual improvement, he does not have arthritis (DMF is not indicated in psoriatic arthritis),7,10 and DMF does not have immunosuppressive effects (which is important as the patient has occasional antibiotics and travels to tuberculosis areas). DMF was initiated in this patient and the dosage was incrementally increased to minimise nausea. Once psoriasis area severity index (PASI) 75 is achieved, the dose could be incrementally and slowly reduced, and later increased again if relapse occurs. Prof van de Kerkhof noted that it is important and remarkable that flexible dosing is possible with Skilarence® using the 30 mg and 120 mg tablets.5,10
To improve tolerability, it is recommended to start Skilarence® treatment at a low initial dose with subsequent gradual increases. Commencing at 30 mg Skilarence® once daily, the dosing can be increased every week by 30 mg until the maximum allowed daily dose of 720 mg (3×2 tablets of 120 mg Skilarence®) is reached. If a particular dose increase is not tolerated, it may be temporarily reduced to the last tolerated dose. If treatment success is observed before the maximum dose is reached, no further increase in the dose is necessary. After clinically relevant improvement of the skin lesions has been achieved, consideration should be given to the gradual reduction of the daily dose of Skilarence® to the maintenance dose required by the individual.10
In the multicentre, double-blind, placebo-controlled, Phase III BRIDGE study11 conducted in four European countries, 704 adult patients were randomised to receive either Skilarence®, Fumaderm® (the active comparator) or placebo in a randomisation schedule of 2:2:1 for a 16 week treatment phase, with a subsequent off-treatment follow-up of ≤12 months. At week 16, approximately 40% of patients achieved a PASI 75 response with Skilarence®, which was significantly higher than placebo (p<0.0001) and noninferior to the active comparator, Fumaderm® (p<0.001).12
It is important to note that the efficacy of DMF increases over time as shown in the multicentre, retrospective, cross-sectional FUTURE study, in which 984 patients were treated with FAE either for 24 months continually or ≥36 months with interruptions (the sum of therapy interruptions totalling not >6 months). Reich et al.13 found that after initial improvement, there is a gradual increase and maximum efficacy is reached after 12 months. The percentage of patients documented as markedly improved or clear was 67.0% after 6 months, 76.2% after 12 months, 77.8% after 24 months, and 82.1% after 36 months of therapy (Figure 1).13
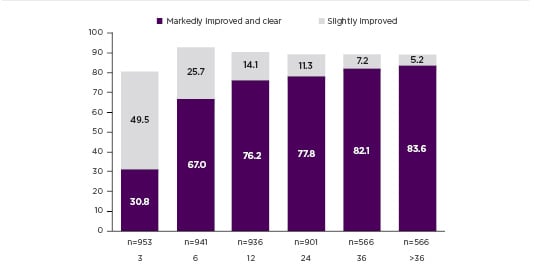
Figure 1: FUTURE Study: Proportion of patients with slightly improved or markedly improved and clear.
Clinical efficacy according to Physician Global Assessment (PGA) during the observation period.
PGA: Physician Global Assessment.
Adapted from Reich et al.13
As anticipated, the adverse event profile of Skilarence® is consistent with Fumaderm®. Some notable side effects observed with DMF include gastrointestinal complaints, flushing, lymphopenia, and reversible peripheral eosinophilia. As discussed previously, the efficacy of DMF increases with time; therefore, appropriate and timely management of adverse events is especially important to achieve the maximum therapeutic effect. In the BRIDGE study, 10% of patients developed lymphopenia and 9% had eosinophilia. These conditions are important to recognise but can be managed adequately by following the clear lymphopenia protocol, that is, assessing lymphocyte counts every 3 months and intervening when the counts fall below 0.7×109/L (Table 1).1,5,10 Adherence to this monitoring protocol may also minimise the risk of development of progressive multifocal leukoencephalopathy (PML), a rare opportunistic infection of the central nervous system caused by the John-Cunningham virus which can be fatal or cause severe disabilities. Only eight patients treated worldwide with DMF have developed PML, related to pronounced and long-standing lymphopenia.10,14,15
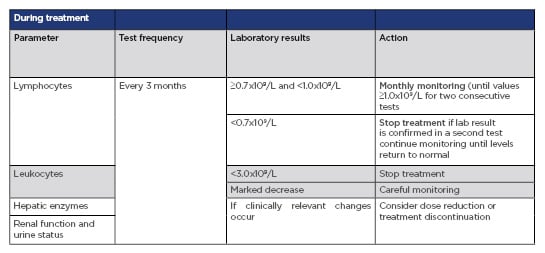
Table 1: Recommended testing and monitoring before and during treatment with Skilarence®
CBC: complete blood count.
Adapted from Skilarence® SmPC.10
One practical consideration regarding DMF is that the indication is not limited by a threshold PASI. DMF only mildly modulates the immune system and is therefore appropriate for patients with cancer and those at risk for infections.15 DMF can be used in combination with phototherapy16 and in patients overtreated with phototherapy. If you follow the guidelines with respect to safety monitoring, this is an effective and safe first-line systemic treatment option.2,15 Prof van de Kerkhof stressed that patient selection is key when deciding which patients will benefit most from DMF. He noted that DMF is not appropriate when you are going for the ‘quick fix’ treatment option and that DMF is not indicated for psoriatic arthritis.7,10
Acitretin is a synthetic aromatic analogue of retinoic acid (vitamin A derivative) and the active metabolite of etretinate, which has been used with success for a number of years in the treatment of severe psoriasis.17,18 The European S3-Guidelines on the systemic treatment of psoriasis vulgaris found insufficient available evidence to generate an evidence-based recommendation for or against the use of acitretin as a monotherapy. Furthermore, based on clinical experience and depending on the most important outcome for the individual patient, a low dose (20–30 mg daily) with respect to tolerability and a high dose (>30 mg daily) with respect to efficacy is suggested. Acitretin is rarely used as a first-line systemic agent in the Netherlands, but in some parts of the world where biologics are difficult to get or get reimbursement for, acitretin in combination with phototherapy may be useful in severe psoriasis. Acitretin may be used in combination with topicals, phototherapy, or methotrexate for chronic plaque psoriasis. Acitretin may be used as monotherapy for pustular psoriasis, where the preferred dosage is 60 mg daily, and for erythrodermic psoriasis, for which the dosage is preferably 25 mg daily. Acitretin may be an appropriate alternative for cases in which systemic therapy is required, and immunosuppressive therapy is contraindicated.5







