Abstract
Alopecia is prevalent among females, categorised as either scarring or non-scarring, depending on the potential for hair follicle regeneration. Various aetiologic factors are implicated in non-scarring alopecia, including genetic predisposition, loss of immune privilege, oxidative damage, and hormonal fluctuations. Telogen effluvium, alopecia areata, and female pattern hair loss are the main causes of non-scarring alopecia in females of all ages. This review covers the aetiology, pathophysiology, and treatment of the most common non-scarring alopecias in females.
Key Points
1. Alopecia is commonly dismissed by the general public as merely a cosmetic concern. However, for many patients, it triggers feelings of low self-esteem, lack of confidence, and even depression or anxiety. This condition, categorised as either scarring or non-scarring, is especially pertinent for females, who frequently experience non-scarring alopecia. This highlights the necessity for a thorough understanding of its causes.2. This narrative review explores the most common causes of non-scarring alopecia. It delves into detailed discussions of telogen effluvium, alopecia areata, and female pattern hair loss, covering their pathophysiological, clinical, and treatment aspects.
3. Non-scarring alopecia in females requires thorough evaluation. Telogen effluvium typically involves several precipitating factors contributing to this limited cause of hair loss. Alopecia areata is considered an autoimmune phenomenon, which can become chronic or recur in some patients. Female pattern hair loss follows a chronic course, with various topical, oral, and procedural treatment options available to reverse or halt its progression.
INTRODUCTION
Scalp hair is often regarded as a symbol of beauty and vitality across many cultures. Consequently, the loss of hair can significantly impact the emotional and psychological well-being of affected individuals. While alopecia (hair loss affecting any area) may be perceived as merely cosmetic by the public, patients frequently experience low self-esteem, lack of confidence, and common feelings of depression or anxiety.1 Therefore, clinicians should consider these psychological impacts when assessing and treating patients with signs of alopecia.
Alopecia can be further categorised into scarring and non-scarring types, with the potential for follicle regeneration serving as the defining differentiator.2 Scarring alopecia, which entails irreparable follicular damage leading to permanent loss, is less common than non-scarring alopecia. Depending on the type of inflammation, primary scarring alopecia is classified as lymphocytic, neutrophilic, or mixed. Clinical and histological diversity make diagnosis and treatment challenging.2,3
Non-scarring alopecia may present with periods of hair loss followed by regrowth; some consider it to be the result of several factors including loss of immune privilege and genetic predisposition.3 Non-scarring alopecias include telogen effluvium (TE), alopecia areata (AA), female pattern hair loss (FPHL), and others.4
Understanding non-scarring alopecia is critical for clinicians since hair loss is a frequent complaint. This paper provides insights into types of non-scarring alopecia affecting females, namely TE, AA, and FPHL.
METHODS
A comprehensive literature search was conducted to identify relevant studies on non-scarring alopecia in females. The search included electronic databases, including PubMed, Scopus, and Web of Science. Keywords used in the search included ‘alopecia’, ‘nonscarring alopecia’, ‘female hair loss’, ‘telogen effluvium’, ‘alopecia areata’, and ‘female pattern hair loss’. The search covered articles published up to 15 October 2023. Inclusion criteria involved studies providing insights into the aetiology, pathophysiology, or treatment of non-scarring alopecia in females, with exclusion criteria applied to articles lacking relevance or focusing on male populations. Methodological rigour was evaluated directly by the authors, and data synthesis was organised around identified themes, presenting a comprehensive narrative on the causes and treatments of non-scarring alopecia. Acknowledging limitations such as language restrictions and the temporal scope of the review, this methodology ensures a thorough exploration of non-scarring alopecia in females.
NON-SCARRING ALOPECIA
In non-scarring alopecia, hair follicles have the potential for recovery and often regain function once triggers such as inflammation, mechanical damage, or chemical exposure cease. Clinically, non-scarring alopecias can be classified as focal (patchy), diffuse, or ‘patterned’.5
Patchy non-scarring alopecia encompasses conditions such as AA, pressure-induced alopecia, tinea capitis, and traction alopecia, where the alopecia is noted in discrete patches. Diffuse non-scarring alopecia includes anagen effluvium, loose anagen syndrome, TE, and certain subtypes of AA, where loss occurs diffusely across the scalp.6 Finally, patterned conditions include FPHL, and trichotillomania.
Alopecia may be a component of autoimmune systemic disorders such as systemic lupus erythematosus. It is imperative to differentiate between diffuse AA and systemic lupus erythematosus.7 In addition, non-scarring alopecia may develop as a result of acute or chronic scalp inflammation in disorders such as psoriasis,8 seborrheic,9 or atopic dermatitis.10
Medications that have been associated with non-scarring hair loss include biologics (e.g., adalimumab, infliximab), chemotherapeutics, tyrosine kinase inhibitors (e.g., erlotinib, imatinib), budesonide, tacrolimus, enoxaparin, and lamotrigine.11
TELOGEN EFFLUVIUM
Follicular activity involves three main phases: anagen, catagen, and telogen. Approximately 90–95% of scalp hairs are in the growth (anagen) phase, where rapid division and differentiation of stem cells leads to follicular growth and hair shaft lengthening. This is followed by the involution (catagen) phase, characterised by apoptosis of follicular epithelium. Finally, the transition to a quiescent or resting state (telogen) involves 5–10% of follicles.12
This cycle occurs in a mosaic pattern so that follicles are not in the same phase synchronously, preventing episodes of mass shedding. On the scalp, anagen may last 2–8 years, telogen 2–3 months, and only 1% of follicles are in the 2–3-week catagen phase. Approximately 50–100 telogen hairs are shed daily.12
TE involves the early transition of anagen hairs to telogen following a stressor, leading to sudden and substantial hair shedding manifesting weeks or months later.13 Typically, an increased number of anagen hairs transition abruptly into catagen, followed by telogen, potentially resulting in a hair shedding rate of up to 35%; compared to the usual 5–10%.13 More recently, the synchronisation of telogen phase termination (teloptosis) has been considered a defining feature of TE, regardless of anagen phase duration.14
Pathophysiology
While the exact mechanism of TE is still not completely understood, theories focus on immediate anagen release, referring to the premature shift of anagen into telogen following stressors.15 This shift could be attributed to the redirection of metabolic priorities in response to circulating stress hormones or cytokines.16 The delayed anagen release theory postulates that a prolonged anagen duration delays telogen onset, and when the anagen stimulus ceases, hair shedding increases as follicles enter telogen. This may be responsible for postpartum TE.17
TE does not have inflammatory histopathologic findings; but rather, increased telogen follicles are the hallmark, and may be accompanied by additional findings if other causes of alopecia are present.18
Clinical Features
Patients with TE observe reduced scalp hair density and severe, abrupt shedding. Loss of less than 50% of hair volume may be noted but it is not a ‘shed to bald’. TE usually manifests 2–3 months following a trigger, with regrowth occurring 6–12 months after resolution. In some cases, individuals may experience a chronic course, with hair loss persisting beyond 6 months.19 Table 1 provides a list of potential TE triggers.
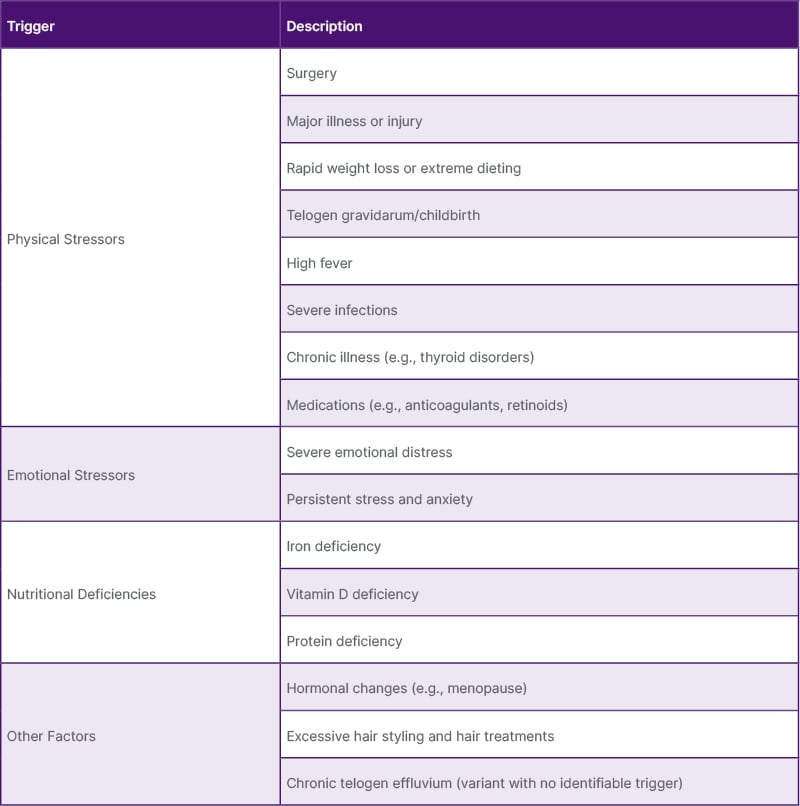
Table 1: Potential telogen effluvium triggers.
Diagnosis
Hair loss distribution is typically diffuse, commonly affecting bitemporal areas, with unremarkable hair shafts and scalp skin. A pull test is positive if, after gently pulling 50–100 terminal hairs, close to 10% of pulled hairs are extracted. This serves as a non-specific diagnostic clue, also seen in FPHL and AA. Clinicians should be aware that TE can affect other hair-bearing areas.
Most patients experience asymptomatic hair loss; however, some individuals may develop pain or discomfort, a condition known as trichodynia. Notably, trichodynia is not unique to TE, and can be observed in FPHL.20
Trichoscopy reveals non-specific findings such as upright regrowing hairs with a tapered end, in a vertical position, and normal hair thickness.21 Notably, trichogram shows an increased telogen count, up to 25%, characterised by club-shaped, depigmented, and degraded epithelial sheaths.22
Laboratory testing is reserved for cases where inciting factors are not identified. This may include a complete blood count, metabolic panel, thyroid-stimulating hormone, ferritin, zinc, folate, vitamin B12, and vitamin D levels. TE is a diagnosis of exclusion, and testing is based on the need to exclude other disorders or to investigate possible underlying disease.23
Treatment
TE is generally self-limited and requires the identification of any associated triggers. Patient counselling, education, and reassurance are essential. Management includes treatment of underlying disease or any associated scalp disorders, dietary rectification, and discontinuation of suspected medications.
Dietary supplementation in patients without nutritional deficiencies is generally discouraged, yet some experts suggest iron supplementation to achieve specific ferritin thresholds. Observational evidence suggests that iron supplementation for patients with TE and ferritin levels below 70 ng/dL reduces the proportion of telogen hairs compared to placebo.24 However, other experts suggest a threshold of 40 ng/dL.25,26
Therapy with topical minoxidil remains controversial because efficacy has not been evaluated. However, clinical observation indicates that it may be useful when shedding begins, to support hair regrowth, or in chronic TE as maintenance therapy, requiring at least 10–12 months of daily usage. Low-dose oral minoxidil has been shown to be effective in chronic TE, with doses ranging from 0.25–2.50 mg taken for at least 6 months, resulting in reductions in hair shedding. Side effects may include postural dizziness, lower extremity oedema, and hypertrichosis in undesired areas (e.g., the face).27
ALOPECIA AREATA
AA is a common non-scarring alopecia characterised by autoimmune dysregulation of the follicular microenvironment. The global incidence of AA is about 2%, with no race or sex difference. All ages may be affected, although it is more common in the third and fourth decades.28
The precise pathogenic mechanism remains elusive. It has been postulated that hair follicles are targeted by the immune system during anagen, causing premature entry into catagen and subsequent telogen, with inhibition of recycling and hence, regrowth.29, 30
Pathophysiology
Immune privilege in hair follicles involves orchestrated immunomodulatory mechanisms to prevent autoimmunity. In AA, this phenomenon is presumptively affected due to the downregulation of protective molecules such as TGF-β, α-melanocyte-stimulating hormone, and IL-1031. Furthermore, increased expression of natural killer group 2D receptor ligands, such as the major histocompatibility complex class I (MHC-I) polypeptide-related sequence A, can lead to local activation of natural killer cells and secretion of interferon (IFN)-γ and IL-15. This stimulates the expression of MHC-I on hair follicle cells, thus allowing for presentation of hidden local antigens.29
Although specific autoantigens implicated have yet to be identified, synthetic epitopes derived from trichohyalin (structural protein) and tyrosinase-related protein-2 may be involved.32 Additionally, activation of the JAK–signal transducer and activator of transcription (STAT) pathway by IFN-γ and IL-15 was discovered to trigger transcription of inflammatory genes and enhance cytokine production.33
Genetic factors have shown an increased risk of AA in families of affected patients, compared to the general population.34 Moreover, twin studies suggest a greater likelihood of the other twin being affected when one monozygotic twin is afflicted, compared to dizygotic twins.35
Histopathologic findings vary depending on disease activity. During active phases, a lymphocytic infiltrate of CD4+ T lymphocytes surround anagen follicles in a honeycomb pattern within and around the bulb. Conversely, in chronic stages, the inflammatory infiltrate is less pronounced, and diagnosis is supported by the presence of eosinophils, lymphocytes, and pigment incontinence, along with an increased catagen-to-anagen ratio.18
Clinical Features
AA commonly presents as well-defined, circular, smooth patches of alopecia developing over 2–4 weeks that affect the scalp and potentially other hair-bearing areas. In some patients, patches can progress to involve the entire scalp (alopecia totalis), or the entire skin surface (alopecia universalis). Although pruritus or pain while touching hair (trichodynia) may precede AA, it is typically asymptomatic.36
Other unusual patterns include ophiasis, where the occipital scalp is affected in a band-like pattern, sisaipho, where AA affects any other scalp area aside from the periphery, and diffuse AA, characterised by sudden, significant diffuse hair loss (AA incognito).37
Exclamation hairs, short broken hairs with the proximal end narrower than the distal, are classic.38 Nail involvement has a prevalence of 10–20% and is associated with severe AA phenotypes. It can precede, coexist, or follow hair loss and manifests as pitting, trachyonichia, onychorrhexis, onycholysis, or onychomadesis.39
Diagnosis
AA commonly presents as asymptomatic hair loss with a tendency for spontaneous resolution and relapse.40 Examination of all hair-bearing areas and nails is recommended. The hair pull test may be used to assess disease activity.41 Clinicians should be mindful of the psychological burden to patients following diagnosis and make appropriate referrals when indicated.42
Trichoscopic findings include black dots resulting from broken hairs, exclamation marks, and Pohl-Pinkus constrictions in hair shafts. In chronic AA, yellow dots are found due to accumulation of sebum in long-standing telogen follicles. Regrowing hairs seem short in a pigtail form.43
Patients with AA may benefit from screening for other autoimmune conditions, mainly thyroid disease. Thyroid-stimulating hormone levels and thyroid autoantibody tests are appropriate in patients with a positive family history.43 Additional screening should be determined by associated signs or symptoms.
Treatment
Treatment considerations rely on careful counselling to incorporate patient preference, disease impact, and evaluation of therapeutic risks. Clinical responses vary, and AA may improve without intervention, especially with limited patchy AA.44
For AA with less than 50% scalp involvement, intralesional corticosteroid injection is first-line therapy. Triamcinolone acetonide injection at 2.5–10 mg/mL is used on the scalp and face in small 0.1 mL aliquots intradermally. The dose should not exceed 40 mg due to the risk of local and systemic effects.45 Treatments can be repeated every 4–6 weeks, with an expectation of regrowth within a few cycles, and should be discontinued once complete regrowth is achieved or if there is no response within 6 months. In patients who cannot tolerate injections, topical corticosteroids are an alternative.46
Systemic corticosteroids are also an option. Oral dexamethasone at 0.5 mg/kg in a 6-week course can induce remission.47 Pulsed therapy has also been explored in extensive or recalcitrant AA. Oral dexamethasone at 0.1 mg/kg/day twice weekly for 24 weeks has improved the Severity of Alopecia Tool (SALT) scores in 40 out of 45 patients in a retrospective multicentric study.48
For scalp hair loss greater than 50%, contact immunotherapy such as diphenylcyclopropenone or squaric acid dibutyl ester may be considered. These therapies exert an immunomodulatory effect, and signs of regrowth are expected after 3 months.49
A systematic review and meta-analysis evaluating diphenylcyclopropenone or squaric acid dibutyl ester for patchy AA, alopecia totalis, and/or alopecia universalis demonstrated a 32.3% (95% confidence interval: 25.3–40.2) rate of complete (90–100%) hair regrowth. Patients with patchy AA showed a higher complete response rate (43%) than those with alopecia totalis or universalis (25%). Potential side effects of topical immunotherapy include dermatitis, urticaria, dyschromia, lymphadenopathy, and vitiligo.50
In recent years, JAK inhibitors (JAKi) have garnered significant attention. Inhibition of the JAK–STAT downstream pathway terminates the T cell-mediated response on the hair follicle by blocking the signalling of inflammatory mediators such as IFN-γ. Baricitinib, ritlecitinib, and deuruxolitinib are oral JAKis that have been used with success in the treatment of AA.51,52
A recent systematic review and meta-analysis evaluating the effectiveness and safety of JAKis in AA concluded that JAKis are associated with lower SALT scores compared to placebo. Furthermore, oral JAKis demonstrated superior outcomes compared to topical agents. In this review, the effectiveness of baricitinib, ritlecitinib, and brepocitinib appeared to be comparable.51
Side effects of JAKis include upper respiratory and urinary tract infections, elevated cholesterol and liver function tests, headaches, and acne, which appear to be temporary and reversible. Despite studies confirming the efficacy and safety of JAKis in AA, additional research is still required to address concerns related to high cost, long-term safety, and potential for relapse after discontinuation.51, 52
Minoxidil is another alternative for AA treatment. Topical daily use at 1–5% has proven efficacious and safe.53 Oral minoxidil at doses of 0.25–5.00 mg 1–2 times daily induces terminal hair regrowth in refractory AA.54 Alternative options often employed as adjuvant therapies include methotrexate and cyclosporine, both of which exhibit a high relapse rate upon discontinuation. Reports on utilisation of hydroxychloroquine, antihistamines, azathioprine, prostaglandin analogues, and calcipotriol exist.55
FEMALE PATTERN HAIR LOSS
Historically, androgenetic alopecia (AGA) is a common cause of hair loss in both males and females. It is characterised by the interplay of hormonal and genetic factors; however, the precise role of androgens in female AGA remains uncertain. Thus, a more accurate and widely accepted term is FPHL.56
FPHL is the most common cause of alopecia in females, and studies indicate that up to 19% of females in the USA exhibit some degree of the condition.57 It has been linked to social anxiety and emotional distress, which prompt females to seek treatment.58
Pathophysiology
Many factors, including genetic, environmental, and hormonal influences have been postulated to trigger follicular miniaturisation in FPHL. This process has not been completely elucidated but seems to involve an alteration in the ratio of terminal to vellus hairs in favour of the latter. Evidence suggests that anagen duration is shortened, decreasing from years to months or weeks.58
Androgens play an important role in male AGA due to the effects of dihydrotestosterone on the hair follicle, leading to hair miniaturisation. A similar mechanism may be associated with FPHL, since females with hyperandrogenism-related disorders (e.g., polycystic ovarian syndrome, ovarian hyperthecosis, or androgen-secreting tumours) can manifest early-onset FPHL.58
Additionally, heightened sensitivity to androgens within follicles is hypothesised to be a contributing factor in hair thinning. The potential influence of oestrogen is also significant, notably in the increased post-menopausal prevalence of FPHL; however, research shows inconsistent results regarding whether oestrogens promote or hinder hair growth.59,60
Genetic factors indicate an association between family history and FPHL risk. Notably, a positive family history has been reported in 40–54% of early-onset cases with normal androgen levels.61 Recently, researchers observed perifollicular inflammation of CD4+ T cells in individuals with FPHL and male AGA; however, specific triggers remain unknown.62
Histopathology shows miniaturisation of follicles and sebaceous pseudohyperplasia. Arao–Perkins bodies, aggregates of elastic fibres in the fibrous streamers, are common in advanced stages.18
Clinical Features
FPHL may present with increased shedding progressing to thinning in a distinct pattern, primarily affecting the frontal and vertex scalp. Like male AGA, the occipital scalp is typically spared. Some patients present with a “christmas tree-like” pattern, where frontal thinning is prominent when hair is parted at the midline, while the frontal hairline is spared.
Diagnosis
Patients may complain of increased scalp visibility, sunburn, or ponytail thinning. This is observed over months to years. Although the course is progressive, some patients report distinct bouts of accelerated hair shedding prior to a visible change in hair density.63
Diagnosis is clinical and includes a thorough medical history, incorporating onset, duration, medication history, and use of nutritional or hormonal supplements. On examination, any signs of hyperandrogenism, such as virilisation, should be noted. A hair pull test is typically negative in long-standing FPHL.64
Evaluation for hyperandrogenism may be warranted because conditions such as polycystic ovarian syndrome, adrenal hyperplasia, and ovarian or adrenal tumours can present with FPHL. Additionally, there is emerging research suggesting associations with metabolic disorders, such as insulin resistance and hypertension.65
Multiple scoring systems exist to classify FPHL. Ludwig’s classification defines hair loss severity over the vertex into three grades, while Olsen’s, also a three-grade system, focuses on the frontal area.66 The Women’s Alopecia Severity Scale (WASS) is a system based on midline hair density loss; it incorporates trichoschopy, which may increase its precision.67
Trichoscopic findings include increased anisotrichosis (variation in diameter of hair shafts), miniaturisation, curved hair shafts, focal atrichia, yellow dots, peripilar brown halos, honeycomb pigmentation areas, and white dots.68 Additional diagnostic tools include phototrichograms, a method that merges epiluminescence microscopy with automated digital image analysis that allows for the estimation of hair quantity and density.69
Scalp biopsy is not mandatory for diagnosis but in selected cases helps exclude scarring alopecia, AA, or TE. Two 4 mm punch samples extending into the subcutaneous fat are recommended for vertical and horizontal processing.70
The relationship between ferritin levels and FPHL is debatable. Certain studies have shown reduced ferritin levels in patients with FPHL in comparison to controls. Similarly, the precise role of vitamin D requires further investigation.71
Treatment
Hair loss reduction and potential regrowth are treatment goals. Clinical responses vary; some patients experience improvement with topical treatment alone, while others require combination therapies.
Ideally, treatment should be initiated before noticeable thinning occurs. This proactive approach leads to more favourable outcomes and increased patient satisfaction. Additionally, it is crucial to address treatment duration, as several months of consistent therapy are typically necessary, and some individuals may require maintenance treatment to preserve results.
Topicals are typically the initial choice, with minoxidil being a well-established first-line treatment. It has vasodilatory properties and activates smooth muscle potassium channels, inducing cell proliferation leading to anagen prolongation.72
Minoxidil is available as a 2% or 5% solution, or 5% foam, all of which are proven efficacious. In a systematic review assessing FPHL treatments, none of the four trials comparing minoxidil 2% with 5% observed a significant difference in efficacy between the two concentrations.73 Solutions are commonly used twice daily, with foam formulations once. Patients may experience increased shedding for the first several weeks. Side effects include pruritus, erythema, hypertrichosis, and dandruff, which may be ameliorated with use of foams with lower propylene glycol concentration.74
Oral therapies may supplement topicals. Antiandrogens, such as spironolactone, are utilised in otherwise healthy patients. Spironolactone reduces testosterone production and blocks androgen receptors. It has demonstrated efficacy as either stand-alone or combination therapy. In an interventional study involving 80 patients with FPHL, 44% experienced hair regrowth, while 44% observed no change in hair density after 12 months of spironolactone 200 mg daily.75 Side effects include menstrual irregularity, postural hypotension, and electrolyte imbalance, especially in the context of renal impairment. Contraception is highly recommended in premenopausal patients.76
Additionally, in a retrospective study of 79 FPHL adults on spironolactone 100 mg daily for 6 months, either as monotherapy or in combination with topical minoxidil or low-level laser therapy (LLLT), all patients either improved or maintained their initial WASS score.77 Recent reports suggest that low doses of spironolactone, up to 50 mg daily, may be effective in the treatment of FPHL if used for an average of 4 months.78
Finasteride partially blocks testosterone conversion to dihydrotestosterone by enzyme inhibition and is considered the standard treatment for male AGA, although its utility in FPHL remains controversial. Evidence indicates that a 1 mg daily dose may not yield significant benefits,79 while others report higher doses as beneficial.80 In the European literature, oral cyproterone acetate is an androgen receptor inhibitor shown to be effective in FPHL with hyperandrogenism but is not available in the USA.81
Other treatment options include platelet-rich plasma (PRP), LLLT, and oral minoxidil. PRP may be a promising option, as regrowth is stimulated through the release of growth factors from platelets. Injection protocols vary, with no consensus or treatment standardisation.82-84 A recent systematic review suggests that PRP injections likely reduce hair loss; however, the authors highlight limitations such as study heterogeneity, small sample size, and potential for publication bias.83
LLLT is another resource which may increase hair density. Possible therapeutic mechanisms include increased adenosine triphosphate production and cellular metabolism, as well as growth factor upregulation, but efficacy evaluation is challenging due to potential biases.85
Recent interest in oral minoxidil has emerged, utilising low doses of 0.25–2.50 mg daily. In a retrospective analysis of 12 females on oral minoxidil, there was a significant improvement in hair density after 24 weeks.86 Other reports suggest that a combination of 0.25 mg oral minoxidil and 25 mg spironolactone decreases shedding and improves hair density.87,88
Side effects of oral minoxidil such as hypertrichosis, hypotension, and tachycardia are dose-dependent and usually resolve with discontinuation. There are reports of rare side effects such as pericardial effusion, pretibial oedema, and arrhythmias.88,89
Surgical intervention may be considered when medical treatments fail. Follicular unit extraction and follicular unit transplantation may be employed, depending on FPHL severity and donor site availability.
CONCLUSION
The prevalence of non-scarring alopecia underscores the importance that clinicians be well-versed in this entity. Recognising hair loss and accurately identifying the type and aetiology of alopecia are mandatory to provide effective care. It is helpful to perform biopsies and relevant laboratory evaluations to exclude mimicking conditions.
Numerous treatment options exist, and ongoing advancements aim to decrease the impact of alopecia. It behoves clinicians to remain updated on recent developments given the disease prevalence. Notably, there remains a scarcity of clinical trials that comprehensively assess causative factors and varying treatment strategies for non-scarring alopecia.






