Abstract
Urticaria is a poorly understood and underestimated clinical condition characterised by the sudden onset of itchy wheals and/or angioedema, which usually resolve within 24 and 72 hours, respectively. It is generally classified as being acute (lasting <6 weeks) or chronic (continuous or intermittent for ≥6 weeks). Chronic urticaria can be further classified as chronic spontaneous urticaria (CSU) and chronic inducible urticaria, appearing in response to specific eliciting factors, such as heat, cold, or sun exposure, or following the application of pressure. Scientific advances have been made in the understanding of pathological mechanisms and treatment, especially associated with CSU. The exact pathological mechanism of how urticaria develops is still not yet fully understood, but the clinical implications on the patients’ quality of life are severe and have been associated with mental disorders and metabolic diseases. The diagnosis of urticaria is based on medical history and clinical manifestations. The treatment pathway begins with the administration of second-generation, nonsedating, nonimpairing histamine 1 receptor antihistamines and, in case of nonresponse, with new-generation biological drugs. The current review presents an update of the pathological mechanisms, diagnosis, clinical management, and treatment of CSU. It also focusses on the future implications of new-generation drugs and their effects on the clinical practice.
INTRODUCTION
Urticaria is a common mast cell-driven disease characterised by wheals and/or angioedema (Figure 1), defined as chronic when symptoms occur continuously or intermittently for ≥6 weeks and as spontaneous when specific eliciting stimuli, such as thermal agents, vibration, cholinergic factors, aquagenic, and delayed pressure, have been excluded as possible triggers.1 When symptoms last <6 weeks, a diagnosis of acute urticaria can be made, and when the above mentioned triggers are identified, a diagnosis of chronic inducible urticaria is assigned.1 Urticaria has a strong impact on patients’ quality of life, and has been associated with anxiety, depression, somatoform disorders, metabolic syndrome, obesity, and sleep difficulties.2-4 Patients can also present associated clinical symptoms, such as joint pain, headache and fatigue, flushing, breathlessness, gastrointestinal symptoms, and palpitations.5 The prevalence of acute urticaria is assessed to be 2-fold higher than chronic urticaria.1 Approximately 50% of cases of acute urticaria are idiopathic (i.e., a specific trigger is not identified) and this condition, which is referred to as acute spontaneous urticaria, can progress to chronic spontaneous urticaria (CSU) in up to 36% of patients.6,7 Chronic urticaria is considered more common in adults, with a peak age of onset between 20 and 40 years, and women are affected twice as often as men.6,8 Two recent studies, however, suggested that the prevalence of chronic urticaria and CSU in the paediatric population is similar to that of the adult population.9,10 Urticaria cases in children and in adolescents might be treated by parents using over-the-counter medications and can possibly explain this underestimation in different studies.9
The average duration of chronic urticaria is 3–5 years11 and its prevalence has been estimated to be 0.5–5.0%,12 while CSU affects approximately 0.5–1.0% of the global population.13
Scientific advances have been made in the understanding of pathological mechanisms and treatment, especially associated with CSU. The current review presents an update of the pathological mechanisms, diagnosis, clinical management, and treatment of CSU. It also focusses on the future implications of new-generation drugs and their effects on the clinical practice.
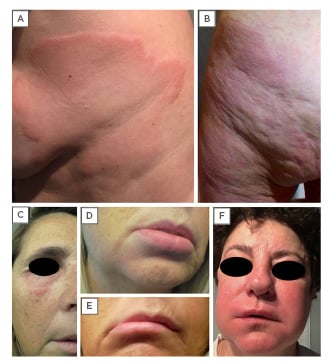
Figure 1: (A-B) Urticaria is characterised by an outbreak of swollen, pale red bumps or plaques on the skin (wheals).
(C-F) Urticaria can also manifest as deep swelling around the eyes, lips, and face (angioedema) that appears
suddenly.
PATHOLOGIC MECHANISMS
The pathogenesis of CSU is complex and many different factors have been proposed as possible triggers including infections, food and drugs allergies, and genetic factors such as human leukocyte antigen Class II alleles associated with autoimmunity and the coagulation cascade. However, these proposed triggers have not been proven to be the causal immunologic mechanism, which today still remains unknown.1 Nevertheless, a strong association is found between CSU and major autoimmune diseases, including autoimmune thyroid diseases, rheumatoid arthritis, Sjögren syndrome, coeliac disease, Type I diabetes mellitus, and systemic lupus erythematosus,14 and there is evidence pointing towards a potential autoimmune aetiology in ≤50% of patients with CSU.15
At histological evaluation, wheals and angioedema present features common to inflammation including the vasodilatation of postcapillary venules, oedema, and a cellular infiltrate characterised by mast cell degranulation and migration of CD4+ T lymphocytes, monocytes, neutrophils, eosinophils, and basophils.16 The oedema develops in the upper- and mid-dermis in the form of wheals, while angioedema involves the subcutaneous or submucosal tissue.1 Cutaneous mast cells play a key role in CSU because their degranulation leads to the relapse of histamine; different proinflammatory cytokines, such as IL-1, IL-4, IL-5, IL-6, IL-8, and TNF-α; platelet-activation factor; vascular endothelial growth factor; matrix metalloproteinase-9; neuropeptides; and other vasoactive substances.5 This is a standard inflammatory mechanism common to many inflammatory diseases.1
Specifically associated with CSU, some authors have demonstrated that upregulation of adhesion molecules for eosinophil cells, in particular P-selectin, alters cytokine expression and microvascular changes in nonlesional skin, and additionally alters the detection of blood basophils in lesional skin exhibiting suppressed IgE receptors, responsible for the release of histamine and upregulation of its release by IL-3.17-19
The recruitment of basophils into wheals results in blood basopenia.20 Following successful treatment, CSU remission has been associated with an increase in blood basophil numbers and IgE receptor-triggered histamine response.20-22 The IgE receptor-triggered histamine response is also observed during anti-IgE treatment.1
It has also been suggested that autoimmunity could be relevant for mast cell activation, specifically for some types of immunological mechanisms.23 Hypersensitivity reactions have been classified into four types (I–IV) according to immunological mechanisms (Gell and Coombs classification of hypersensitivity reactions).24 Type I hypersensitivity is due to the presence of an allergen that binds IgE, present on mast cell and basophil surfaces. This link induces the degranulation of the mast cells and basophils, and the release of mediators. Type II is mediated by antibodies, typically IgG or IgM, which link either IgE and/or high affinity IgE receptor (FcεRI) on mast cells and basophils or low-affinity IgE receptor (FcεRII) on eosinophils leading to vasoactive mediator release.23
The coagulation cascade has also been hypothesised as a possible CSU immunologic mechanism.25 In fact, it has been observed that some CSU subjects have high levels of D-dimer, secondary to activation of the coagulation cascade by the activated eosinophils hyper-expressing tissue factor and other activated coagulation factors that amplify the release of histamine from mast cells and basophils.26
Another possible immunological mechanism recently studied is the endocrine abnormalities of fatty tissue in overweight CSU patients. The fatty tissue may lead to the production of several adipokines that directly target human mast cells and also play a role in endothelial inflammation leading to the production of atherosclerotic plaque.27 Chronic inflammatory skin diseases are known to be a risk factor for metabolic syndromes, and patients with metabolic syndromes and CSU exhibit high levels of prothrombin fragment 1+2, D-dimer, and inflammatory markers such as IL-6, IL-1, TNF-α, and C-reactive protein.28
DIAGNOSIS AND CLINICAL MANAGEMENT
According to the European Academy of Allergology and Clinical Immunology (EAACI), the European Union (EU)-founded network of excellence, the Global Allergy and Asthma European Network (GA²LEN), the European Dermatology Forum (EDF), and the World Allergy Organization (WAO) guideline, diagnostic work-up starts from medical history and physical examination of the patient.1 It is important to know the time of onset, timing, frequency, symptom duration, the features of the disease (i.e., wheals only, or wheals and angioedema), characteristics of the lesions (shape, size, site, distribution, and pattern of recurrence), other associated symptoms, familial disease history, and response to previous therapies used.1,12
The identification of known potential causes and/or possible triggers (e.g., food, medications, physical stimuli, infections, insect stings, and stressful occurrences) of urticaria is essential. Current drug assumption, especially nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors, should also be established, because these drugs have been associated with the aggravation of pre-existing CSU.1 Food avoidance with elimination diets is not helpful for CSU, while alcohol should be sidestepped because it can significantly exacerbate this condition.12
The EAACI/GA²LEN/EDF/WAO guideline recommended laboratory tests such as differential blood count, erythrocyte sedimentation rate, and C-reactive protein.1 Furthermore, concomitant autoimmune disorders, thyroid dysfunction, and acute or chronic bacterial (e.g., Helicobacter pylori), viral (e.g., hepatitis virus), parasitic (e.g., Anisakis simplex), or fungal infections need to be investigated.1 Screening for neoplastic diseases is not recommended but, if there are atypical features, assessment of serum tryptase, complement levels, and serum protein electrophoresis should be considered and a skin biopsy can be performed.1,12
Recent studies have highlighted that CSU may in some cases be associated with elevated BMI, obesity, diabetes, hyperlipidaemia (high levels of serum total cholesterol, triglycerides, low density lipoprotein, and decreased high density lipoprotein), arterial hypertension, metabolic syndrome, and gout.28,29
Differential diagnoses from other conditions in which wheals and angioedema can occur, such as exercise-induced anaphylaxis, auto-inflammatory syndromes, urticaria pigmentosa, urticarial vasculitis, or hereditary angioedema, are made clinically.
Several biomarkers have been investigated in association with CSU activity. Currently, the autologous serum skin test (ASST) and the basophil activation test (BAT) are the most commonly available auto-antibodies screening tests.1 ASST is a relatively simple in vivo nonspecific screening test in which the autologous serum is injected back into the patients’ skin intradermally to evaluate serum auto-reactivity, mostly due to any type of endogenous proinflammatory or wheal-inducing factors.30 A positive ASST has been associated with prolonged disease, which is poorly responsive to routine therapy, and related to a delayed response to omalizumab.31 BAT is an in vitro test that assesses the histamine upregulation or release of activation markers of donor basophils following stimulation from CSU patients’ serum;32 therefore, it helps to co-assess disease activity in CSU patients and a negative BAT is correlated with a better response to omalizumab.33 Indirect BAT is a safe and reliable diagnostic tool which is also helpful in monitoring treatment, however it is usually not routinely available in daily clinical practice.32,34-36 Another test proposed by Asero et al.37 is the autologous plasma skin test (APST). Although the APST cannot be considered a screening test for histamine-releasing autoantibodies, it has recently been shown to a have a greater positive predictive value than ASST and has been correlated with antinuclear antibody positivity and angioedema.38
Moreover, D-dimer is related to disease activity in CSU patients due to the activation of the coagulation cascade and it seems to be the most promising biomarker.25,26 This observation was confirmed by Kolkhir et al.,39 who suggested that the evaluation of not only D-dimer but also fibrinogen, C-reactive protein, and erythrocyte sedimentation rate should be considered before starting treatment, because high levels of these markers may predict an unsatisfactory therapeutic response.
However, none of these biomarkers are currently implemented routinely in clinical practice. The still low level of evidence to support the available biomarkers is probably due to the wide variability and heterogeneity in the data collected from the published studies, which often show differences in methodology, design, selection of patient populations, and/or data analysis.
CSU patient management begins with the compilation of patient-reported scoring.40 The most frequently utilised scoring system is the 7-day Urticaria Activity Score (UAS7).1,40 The UAS7 is based on the patient self-assessment of key urticaria signs and symptoms (wheals: 0 = none; 1 = mild [<20 wheals/24 h]; 2 = moderate [20–50 wheals/24 h]; 3 = intense [>50 wheals/24 h or large confluent areas of wheals] and pruritus: 0 = none; 1 = mild [present but not troublesome]; 2 = moderate [troublesome but does not interfere with sleep]; 3 = severe [sufficiently troublesome to interfere with normal daily activity or sleep]) once a day for 1 week.
The UAS7 is the sum of the recorded scores over the period of 7 consecutive days, so disease activity and eventually response to treatment can be determined. The sum of score is 0–6 for each day with a maximum of 42 if summarised for a week. Additionally, for patients with recurrent angioedema, the EAACI/GA²LEN/EDF/WAO guideline also suggested the use of the Angioedema Activity Score (AAS).1 The AAS consists of five items regarding the characteristics of angioedema to have occurred in previous 24 hours.41 A score between 0 and 3 is assigned to every answer field. The question scores are added up to produce a daily score. Daily AAS can be summed to give 7-day scores, 4-week scores, and 12-week scores.41 The minimum and maximum possible AAS scores are 0–15 (daily), 0–105, 0–420, and 0–1,260, respectively.
To evaluate the impact of urticaria on patients, the Urticaria Control Test that assesses patient’s disease status (the cut-off value for a well-controlled disease is 12 of 16 possible points), and specific disease quality of life questionnaires (the Chronic Urticaria-Quality of Life Questionnaire [CU-Q2oL] and Angioedema-Quality of Life Questionnaire [AE-QoL]) can also be used.1,40
TREATMENT
The aim of pharmacological treatment is to obtain complete symptom relief. EAACI/GA²LEN/EDF/WAO guideline suggests regular administration of second-generation, nonsedating, nonimpairing H1-receptor antihistamines as first-line symptomatic treatment for urticaria because of their good safety profile.1 Compared to the first-generation antihistamines, these antihistamines have greater receptor specificity, lower penetration of the blood–brain barrier, and are less likely to cause drowsiness or psychomotor impairment.10,42 First-generation antihistamines should therefore be avoided due to their sedating, impairing, and anticholinergic side effects, while H2-receptor blockers are not felt to be of benefit in the treatment of urticaria.43
In nonresponders (adult or paediatric patients), the second-line treatment is the up-dosing of the second-generation H1-receptor antihistamines by as much as 4-fold. The leukotriene receptor antagonists, in particular montelukast, can be used as an add-on to second-line treatment in H1-antihistamine refractory CSU, but their administration is not recommended by the EAACI/GA²LEN/EDF/WAO guideline.1
For patients (aged 12 years and older) with CSU who have not responded to four-times the standard dose of second-generation H1-receptor antihistamines, omalizumab, a humanised monoclonal anti-IgE antibody, as add-on therapy is now considered the third-line treatment. Omalizumab was the first biologic agent approved by the U.S. Food and Drug Administration (FDA) for CSU. This drug has been widely proven to be very effective and well-tolerated in patients with antihistamine-refractory CSU.1 Omalizumab binds to free IgE at the fragment crystallisable region (Fc region) preventing interaction with FcεRI receptor on mast cells and basophils. However, the exact mechanisms for the therapeutic effects of this drug for CSU remain unclear. Both 150 and 300 mg of omalizumab injected subcutaneously every 4 weeks have been shown to be effective for refractory CSU (the licensed dosage in Europe is 300 mg, while in the USA this is either 150 or 300 mg).40 Dosage is currently recommended independently of total serum IgE count or patient body weight. Instead, only the dosage of 300 mg every 4 weeks has been proven to be effective in case of angioedema.44
Alternative dosages (off-label) of omalizumab have been used successfully in refractory CSU and reported in small case series: low doses of omalizumab (150 mg every 4 weeks) for long-term management of patients following initial therapy, and high dosages (450 or 600 mg every 4 weeks) for partial or nonresponders.40 Omalizumab nonresponders are considered those with no symptom control after four doses of omalizumab 300 mg every 4 weeks, because the response rate is similar to placebo after this 16-week period.45 To date, strategies or duration of omalizumab therapy, once disease control is optimised, has not found a universal agreement.
For all patients with wheals and angioedema, corticosteroid administration, in particular prednisone (dosage 0.3–0.5 mg/kg/day), over restricted periods of time (typically ≤10 days) can be prescribed as add-on treatment.
Finally, the fourth-line treatment (if there is no response to omalizumab within 6 months, or if the condition is intolerable) is cyclosporine A (CsA). CsA inhibits the production of IL-2, IL-3, IL-4, and TNF-α in lymphocytes and inhibits the IgE-mediated release of histamine from mast cells. High doses of CsA and long duration treatment are associated with adverse events such as abdominal pain, nausea, vomiting, paresthesia, headache, hirsutism, elevated serum creatinine, and hypertension; however, these effects resolve after reducing dose.46 Nevertheless, CsA should be avoided in patients with chronic kidney disease or poorly controlled hypertension. CsA at the dose of 3–5 mg/kg/day has been shown in small, double-blind, randomised controlled trials to be effective in patients with CSU who do not adequately respond to antihistamines.47,48 During CsA treatment, given the significant side effects, the blood pressure, renal function, and serum cyclosporine levels should be monitored regularly. Simplified stepwise algorithm for the treatment of CSU adapted from the EAACI/GA²LEN/EDF/WAO guideline is summarised in Figure 2.
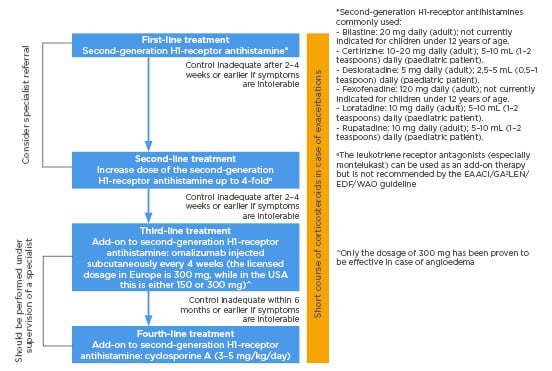
Figure 2: Simplified stepwise algorithm for the treatment of urticaria adapted from the EAACI/GA²LEN/EDF/WAO guideline 2018.
OTHER THERAPEUTIC OPTIONS (Table 1)
Phototherapy reduces the number of cutaneous mast cells in the superficial dermis and it has been used for the treatment of antihistamine-refractory corticosteroid-dependent CSU in combination with antihistamines for periods between 1 and 3 months, but published data are still limited.49,50 Recently, 50 patients with steroid-dependent CSU were randomised to receive either narrowband ultraviolet B (NB-UVB) or psoralen plus ultraviolet A phototherapy in addition to licensed doses of antihistamines for 90 days.51 The reduction in symptoms was maintained in both groups during a 90-day post-treatment observation period, but NB-UVB phototherapy was found to be statistically better than psoralen plus ultraviolet A at different time points. Bishnoi et al.51 proposed the combination of antihistamines with NB-UVB prior to third-line treatment with omalizumab.
Intravenous Ig (IVIg) has been used successfully for the treatment of antihistamine-refractory CSU due to its immunoregulatory effects. Its mechanisms for the immune modulation and anti-inflammatory actions include Fc receptor blockade (IVIg blocks FcεRI activity on mast cells, which prevents IgE binding and degranulation), inhibition of complement deposition, enhancement of regulatory T cells, inhibition or neutralisation of cytokines and growth factors, accelerated clearance of autoantibodies, modulation of adhesion molecules and cell receptors, and activation of regulatory macrophages through the FcγRIIb receptor.52 However, due to the high cost, prolonged infusion times, and limited data on efficacy, the clinical use of IVIg is somewhat limited and alternative biologic agents should be considered.
CSU patients have been proven to have an upregulated TNF-α in the lesional and nonlesional skin.53 TNF-α inhibitors (etanercept, infliximab, and adalimumab) have been reported to be effective in the treatment of CSU and may be a therapeutic option to those who have failed other alternative therapies.53,54 However, no head-to-head studies have been performed to date and these drugs may be limited by their increased risk for infections, including tuberculosis and fungal infections, along with an increased risk for lymphomas and other malignancies.
Rituximab, a mouse-human chimeric anti-CD20 monoclonal antibody, induces B-cell depletion by targeting the CD20 antigen on the B lymphocytes. This mechanism results in inhibition of autoantibody production and some promising results have been demonstrated in patients with CSU;55 however, given the lack of randomised, double-blind, placebo-controlled trials, rituximab is not licensed for the treatment of antihistamine-refractory CSU.
Future therapeutic options currently under investigation include new-generation biological drugs. Ligelizumab, a humanised IgG1κ monoclonal antibody targeting the third heavy chain constant region domain of IgE, is similar in function to omalizumab, but has been proven to bind free IgE with greater affinity.56 A Phase IIB dose-finding trial57 evaluated the efficacy and safety of this drug compared with placebo and omalizumab, showing complete control of symptoms in a higher percentage of patients with ligelizumab therapy of 72 mg or 240 mg.58 Currently, an extension study59 is investigating the long-term safety of this drug in CSU patients who completed the trial, remained in the follow-up period for at least 32 weeks, and had an active disease (UAS7≥12).
Another humanised IgG1 monoclonal antibody is quilizumab, which targets the M1 prime segment of membrane-expressed IgE resulting in diminished IgE-switched B cells and plasmablasts. The effects appeared to last up to 6 months after completion of therapy,60 however, further development of this drug has been discontinued.
Other agents are currently under experimentation in clinical trials. Bruton tyrosine kinase is a nonreceptor tyrosine kinase that transmits signals crucial for B-cell development and its genetic deletion causes B-cell immunodeficiency.61 Although the role of B cells in urticaria is not well understood, it is believed that bruton tyrosine kinase inhibitors could potentially play a role in refractory CSU management. GDC-0853, a bruton tyrosine kinase inhibitor, is currently under investigation in a Phase IIA multicentre, randomised, double-blind, placebo-controlled pilot study62 that is evaluating the efficacy, safety, and pharmacodynamics of this drug compared with placebo in individuals with anthistamine-refractory CSU.
Spleen tyrosine kinase upregulates transcription factors that are responsible for the synthesis and degranulation of proinflammatory mediators and its expression increases in certain subsets of patients affected by CSU.63-65 GSK2646264, a topical spleen tyrosine kinase inhibitor, is currently being tested in a randomised, double-blind, single and repeat ascending trial in order to determine its efficacy in patients with CSU and cold urticaria.66
Inhibition of IL-1β may modify the clinical course of urticarial lesions in patients affected by CSU and various studies are underway in order to elucidate the role of IL-1 inhibitors in CSU.67 Canakinumab, a fully human anti-IL-1β antibody, is currently under investigation in a Phase II randomised, double-blind, placebo-controlled, single-centre study evaluating the use of this drug compared with placebo in CSU patients.68 Finally, inhibition of chemoattractant receptor-homologous molecule expressed on TH2 cells could reduce the frequency and severity of urticarial lesions because of its anti-inflammatory properties. CSU patient eosinophils overexpress this prostaglandin D2 receptor.69 A Phase IIA, randomised, placebo-controlled, double-blind study is evaluating the efficacy of AZD1981, a prostaglandin D2 receptor antagonist, as a potential therapeutic option in CSU.70
Further investigations are needed for identifying strategies for the prevention and symptomatic treatment of CSU, identification of the best therapy, and development of new drugs. Moreover, the exploration of novel therapeutic targets can help to better understand the aetiopathogenesis of the disease.
CONCLUSIONS
There is currently a low level of evidence on the exact pathological mechanism in CSU, and as a result clinical and diagnostic indications have not recently changed. New-generation treatment options should be available in the near future and seem promising. Future studies should investigate personalised treatment, with recommended dosages considering disease severity and treatment responsiveness.






