Abstract
Intracardiac echocardiography (ICE) has been adopted for use in catheter ablation because of its multiple real-time applications. It can be used to guide transseptal puncture, visualise cardiac and great vessel anatomy and physiology, and provide safe guidance and positioning of catheters within the left and right side of the heart. These benefits have the potential to prevent procedural complications when ICE is used. The objective of this review is to highlight three recently published studies that add to the existing body of clinical evidence on the benefits of ICE use during ablation. Collectively, these studies support the conclusion that adoption of ICE into ablation workflows results in improvements in procedural and safety outcomes, which are likely to translate into long-term clinical and economic benefits for patients, providers, and healthcare systems.
INTRODUCTION
Catheter ablation is a well-established, safe, and effective treatment option for patients with atrial fibrillation (AF) and ventricular tachycardia (VT).1 AF is the most common cardiac arrythmia. The prevalence of AF is predicted to grow to between 6.0 and 12.0 million people in the USA by 2050, and to 17.9 million people in Europe by 2060.2-4 Those affected by AF have an increased risk of heart failure, stroke, and mortality, as well as lower quality of life.5,6 The incidence of VT is not well quantified, but it is associated with high morbidity.7,8 Subsequently, AF and VT place a substantial economic burden on patients, caregivers, and healthcare systems.9
Over time, imaging modalities in catheter ablation have evolved, yielding substantial improvements in navigation, lesion formation, and procedural safety. Catheter ablation procedures, however, have relied upon imaging modalities such as fluoroscopy, CT, MRI, and transoesophageal echocardiography, which all have limitations.1,10-15 Fluoroscopic imaging offers limited insight into endocardial anatomy and localisation of the catheter, and exposes patients and healthcare providers to harmful ionising radiation.10,11 While all the aforementioned modalities are compatible with three-dimensional (3D) electroanatomical mapping (EAM), CT and MRI imaging do not provide real-time visualisation or monitoring of complications, and transoesophageal echocardiographic imaging may not provide high resolution images.1,12-15
Intracardiac echocardiography (ICE) addresses many of the limitations of other imaging technologies for catheter ablation. During catheter ablation, ICE facilitates low fluoroscopy use, produces high-resolution images, and offers real-time visualisation of cardiac structures, while providing multiple real-time applications.12,16-18 Currently, two types of ICE imaging systems are commercially available: the mechanical ultrasound catheter with a radial or rotational imaging system and the electronic phased-array catheter with a sector imaging system.16 ICE is compatible with 3D EAM systems: CARTO® System (Biosense Webster, Irvine, California, USA), EnSite NavX® (Abbott, Lake Bluff, Illinois, USA), and Rhythmia® (Boston Scientific, Marlborough, Massachusetts, USA).3 ICE has also become the standard imaging method in centres that perform therapeutic procedures with catheters for patients with structural cardiopathy.12
INTRACARDIAC ECHOCARDIOGRAPHY TECHNIQUE
Typically, the ICE catheter is introduced through the femoral vein and into the right atrium to provide a ‘home view’, in combination with fluoroscopic images that provide a baseline assessment of the cardiac structures.16 Other views can be captured (e.g., septal, long, and short axis views) after the home view image is obtained.19
In AF ablation, the ICE catheter provides visualisation of the interatrial septum and the oval fossa, which is the safest point for the transseptal puncture.20 It also draws contours of the left atrium and of the pulmonary vein anatomy from the right side.16 The ICE catheter is usually integrated with other imaging modalities to provide information about catheter positioning and assessment of lesion formation.19 ICE provides real-time monitoring of the mapping catheter position and catheter-to-tissue contact of the ablation catheter. Furthermore, visualisation of microbubble generation with ICE can be used to guide energy delivery and optimise lesion formation.17 A summary of the clinical value that ICE provides during the access (including transseptal puncture), mapping, and ablation phases of AF catheter ablation is presented in Box 1.
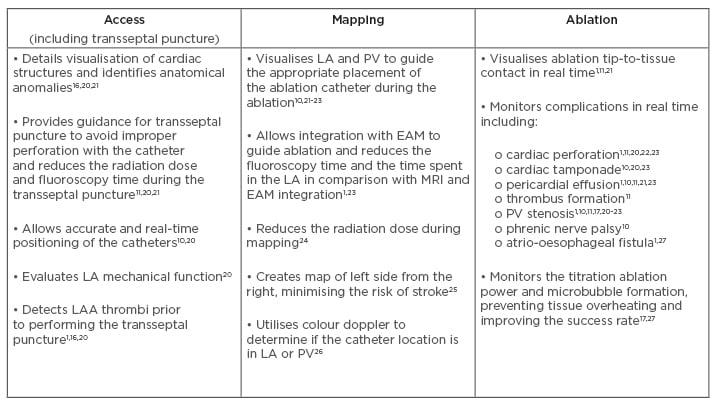
Box 1: Clinical value of intracardiac echocardiography by ablation procedural phase in atrial fibrillation catheter ablation.
AF: atrial fibrillation; EAM: electroanatomical mapping; LA: left atrium; LAA: left atrial appendage; PV: pulmonary vein.
In addition, ICE can be used in VT ablation to visualise catheter position when ablating arrhythmias close to cardiac structures or arteries.19 In the ablation of both AF and VT, ICE provides continuous monitoring that is used to prevent or treat potential complications.19
USE OF INTRACARDIAC ECHOCARDIOGRAPHY IN ATRIAL FIBRILLATION ABLATION
ICE integrates real-time imaging with 3D EAM systems to enhance the clarity of anatomical images, reducing the need for fluoroscopy. Numerous clinical studies have demonstrated substantial reductions in fluoroscopy time and fluoroscopy dose in AF ablation with the use of EAM with ICE, as opposed to using EAM without ICE, with no additional adverse safety events.24,28-30 For example, a randomised controlled trial including 60 AF patients randomised to EAM or ICE plus EAM showed a significant, 22% reduction in fluoroscopy time and a significant, 125 Gy/cm2 reduction in fluoroscopy dose with ICE plus EAM versus EAM alone.24 Reducing fluoroscopy is critical because its exposure leaves patients and healthcare professionals vulnerable to potentially harmful ionising radiation, which is associated with an increased risk for cancer development.31-34
USE OF INTRACARDIAC ECHOCARDIOGRAPHY IN VENTRICULAR TACHYCARDIA ABLATION
Transseptal puncture during VT ablation often requires prolonged fluoroscopy to validate needle positioning. Clinical studies demonstrate that use of ICE to visualise the atrial septum can significantly reduce both fluoroscopy duration and dose, decreasing radiation exposure for healthcare professionals and patients.30,35 A retrospective analysis of a mixed cohort of 120 ablation patients (including VT ablation) reported catheter ablation with ICE plus EAM (no fluoroscopy) was feasible, with no additional safety concerns, when compared to catheter ablation with fluoroscopy and EAM.33
LIMITATIONS OF INTRACARDIAC ECHOCARDIOGRAPHY USE
Cost, image resolution, and learning curve are limitations of ICE. There can be a high upfront cost associated with an ICE catheter. In addition, there can be increased costs associated with hiring staff, if ultrasound imaging procedures are adopted broadly in electrophysiology labs. The potential for decreased procedural time, fluoroscopy use, and complications, however, may position ICE to be a cost-effective option in the long term.36 Use of current ICE imaging for intracardiac tissues, complex structures, and scars may not be optimal for visualisation. Upcoming novel four-dimensional ICE technology may result in superior image acquisition and allow for a full spectrum of imaging capabilities when integrated with EAM systems. Additionally, there is a learning curve associated with use of ICE. Although ablation procedures incorporating ICE require an experienced echocardiographer, the learning curve for use of ICE imaging varies.
The duration of the learning curve is dependent on the operator’s previous experience in ultrasound and the centre’s available expertise in ICE. Moreover, if a centre aspires to perform more complex ICE procedures, the learning curve may be greater than for simple applications of ICE.
NEW CLINICAL EVIDENCE SUPPORTING USE OF INTRACARDIAC ECHOCARDIOGRAPHY IN ABLATION OF CARDIAC ARRHYTHMIAS
In the months preceding the writing of this article, several novel studies have published findings that demonstrate improved outcomes associated with the use of ICE during ablation of arrhythmias (Table 1).37-40 The following sections will describe in more detail the key findings from these new studies, which build on the current clinical evidence base supporting ICE use in catheter ablation of cardiac arrhythmias.
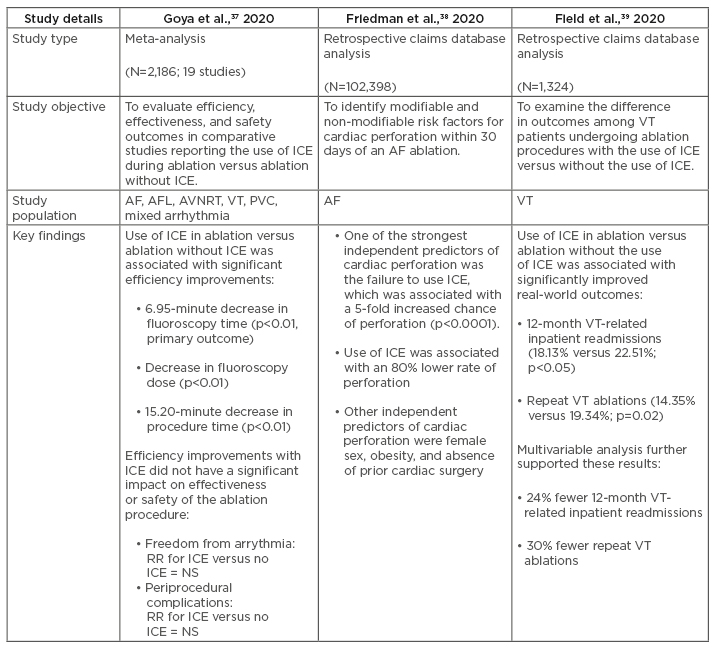
Table 1: Summary of recent clinical studies examining the clinical value of intracardiac echocardiography in ablation of cardiac arrhythmias.
AF: atrial fibrillation; AFL: atrial flutter; AVNRT: atrioventricular nodal re-entry tachycardia; ICE: intracardiac echocardiography; NS: non-significant; PVC: premature ventricular contractions; RR: relative risk; VT: ventricular tachycardia.
Meta-Analysis of Efficiency, Effectiveness, and Safety Outcomes in Ablation with Intracardiac Echocardiography versus Ablation without Intracardiac Echocardiography37
Although previous studies have individually reported that ICE reduces dependency on fluoroscopic imaging,24,28-30 a recently published meta-analysis is the first to systematically compare outcomes in ablation amongst procedures that use ICE technology versus those that do not.37 This systematic review and meta-analysis, which includes 19 comparative studies (N=2,186), found that ablation of cardiac arrhythmias using ICE is associated with significant reductions in fluoroscopy time (primary outcome) (Hedges’ g: -1.06; 95% confidence interval [CI]: -1.81 to -0.32; p<0.01), fluoroscopy dose (Hedges’ g: -1.27; 95% CI: -1.91 to -0.62; p<0.01), and procedure time (Hedges’ g: -0.35; 95% CI: -0.64 to -0.05; p=0.02) compared to ablation without ICE. Fluoroscopy time ranged from 3.9±2.6 to 77.0±18.0 minutes with ICE versus 5.9±3.4 to 83.0±24.0 minutes without ICE. Procedure time ranged from 82.0±20.8 to 232.0±53.0 minutes with ICE versus 72.1±19.0 to 250.0±66.0 minutes without ICE. Mean difference (MD) analysis demonstrated a 6.95-minute average reduction in fluoroscopy time (MD: -6.95; 95% CI: -11.25 to -2.66; p<0.01) with ICE versus without. The average reduction in procedure time was 15.2 minutes (MD: -15.2; 95% CI: -26.40 to -4.00; p<0.01) with ICE versus without. Greater reductions in fluoroscopy time were seen when a sensor-based ICE catheter (i.e., SOUNDSTAR® Catheter [Biosense Webster]) was used, corresponding to a 12.7-minute reduction versus ablation without ICE.
Improvements in efficiency during ablation with ICE did not compromise effectiveness or safety outcomes. Similar (nonsignificant) rates of acute success and freedom from arrhythmia were associated with catheter ablation with the use of ICE versus without. Although there was a trend towards lower periprocedural complications (excluding venous access) with ICE versus without ICE, the decrease in complications was not significant, likely due to low event rates.
Increased fluoroscopic and procedural efficiency during ablation with ICE result in several important, improved outcomes. Reduction in fluoroscopy minimises radiation risks for patients, operators, and staff, and helps to reduce the incidence of occupational orthopaedic injuries for healthcare professionals that are associated with the prolonged use of lead. In addition, reduction in procedure time is likely to be associated with reductions in procedure cost.
Retrospective Analysis of the Predictors of Cardiac Perforation in Catheter Ablation of Atrial Fibrillation38
A recently published retrospective study from the USA was conducted to identify contemporary modifiable and non-modifiable risk factors for cardiac perforation within 30 days of AF ablation in fee-for-service Medicare beneficiaries. Cardiac tamponade, although rare, is one of the most frequent causes of periprocedural death in AF patients.1 One of the most common causes of cardiac perforation leading to cardiac tamponade during AF ablation is misdirected transseptal punctures.1
In this large dataset (N=102,398), among patients ≥65 years of age who underwent AF ablation from 1st July 2013 to 31st December 2017, cardiac perforation occurred in 0.61% (n=623) of patients within 30 days of the index ablation procedure. Use of ICE was associated with an 80% lower rate of perforation, whereas risk of perforation was increased 5-fold if ICE was not used. Other independent predictors of cardiac perforation were female sex, obesity, and absence of prior cardiac surgery.
Safer realisation of transseptal punctures are achieved with the use of ICE through the direct visualisation of ‘tenting’ and needle/catheter tip trajectory, which may reduce the risk of potentially fatal complications such as puncture to the aorta or left atrial wall.20 The findings of this study, which is the single largest study assessing predictors associated with cardiac perforation, suggest significant safety benefits associated with the use of ICE in AF procedures. It may also warrant increased adoption of ICE and broader recommendations regarding its use for minimising the risk of perforation and facilitating continued improvement in procedural safety during ablation.
Real-World Analyses of the Value of Intracardiac Echocardiography in Ventricular Tachycardia Ablation39,40
Although previous studies have shown improved outcomes in VT ablation with ICE,30,35 the impact of its use in the real world has not been thoroughly investigated. Recently, a study was published that used real-world data from a retrospective claims database to examine differences in outcomes including readmissions and repeat ablations among VT patients receiving ablation with ICE versus those who had ablation without ICE.39 Patients with a history of implantable cardioverter defibrillator/cardiac resynchronisation therapy who underwent VT ablation were identified using the 2008–2017 IBM™ MarketScan Commercial and Medicare Supplemental Databases. Two cohorts were established (one for use of ICE and one without) and patients were matched using propensity score matching (N=1,324). Improved outcomes were observed among VT patients who underwent catheter ablation with ICE compared to patients who received ablation without ICE, including significantly lower rates of 12-month VT-related readmission (18.13% versus 22.51% for ICE and without ICE, respectively; p<0.05) and repeat VT ablation (14.35% versus 19.34% for ICE and without ICE, respectively; p=0.02). Multivariable analysis showed that patients with VT who underwent ablation with ICE had a 24% lower risk of VT-related readmission (odds ratio: 0.76; 95% CI: 0.58–0.99) and a 30% lower risk of repeat VT ablation (odds ratio: 0.70; 95% CI: 0.52–0.93) compared to patients who underwent ablation without the use of ICE. Similar results were observed in a prespecified subgroup analysis of patients with ischaemic heart disease. This real-world study suggests that improvements in the effectiveness of VT ablation with ICE can potentially be translated to resource optimisation and economic savings by reducing postablation resource utilisation.
Similar outcomes were demonstrated in another recent claims database analysis study examining ICE outcomes after VT catheter ablation.40 In this study, 2,820 patients with a primary diagnosis of VT undergoing outpatient catheter ablation were identified using the 2008–2017 Centers for Medicare and Medicaid Services (CMS) Standard Analytical Files database. Based on propensity matching, 2,152 patients were included: 1,076 patients in each of the ICE and without ICE groups. Patients in the ICE group had a 24% lower risk of all-cause readmission (hazard ratio [HR]: 0.76; 95% CI: 0.67–0.86), 24% lower risk of cardiovascular-related readmission (HR: 0.76; 95% CI: 0.66-0.87), and 20% lower risk of VT-related readmission (HR: 0.81; 95% CI: 0.67–0.97) compared with patients in the without-ICE group. No significant difference in repeat ablation or complications was observed between the two groups. VT ablation patients with transseptal puncture and the use of ICE had significantly lower risk of 12-month all-cause inpatient readmission (45.89 versus 63.63%; p<0.0001), cardiovascular-related readmission (32.90 versus 53.25%; p<0.0001), and VT-related readmission (15.15 versus 26.84%; p<0.0001) compared with patients in the without-ICE group. These findings demonstrate the significant value that ICE provides, particularly for those VT patients requiring transseptal puncture.
LIMITATIONS AND FUTURE STUDIES
There are several limitations associated with the new studies described. Several of the studies described in this review used data derived from medical insurance databases. These data provide limited information regarding procedural complexity, which has a bearing on comparisons examining the procedural efficiency of ICE. Although propensity score matching is used to minimise the effect of confounders and selection bias, the effect of unmeasured confounders (e.g., procedural complexity, operator experience, ablation technique, and utilisation of contact force-sensing catheters) cannot be excluded. The difference in commercial insurance coverage might also affect the decision to use ICE in ablation.
There are also several limitations associated with the meta-analysis. One limitation is that it incorporated studies that spanned over a very long period of time. The comparison of procedural safety and efficacy outcomes in different periods can be confounded due to the variation in patient characteristics, arrhythmia complexity, procedural techniques, ablation catheters, and operator experience. Moreover, because the rate of complications was low among included studies, a statistical difference in the incidence of complications among procedures that use ICE versus those that do not use ICE could not be definitely established.
While these studies highlight the role of ICE in improving outcomes in ablation procedures, the potential mechanisms or attributes of ICE that contribute to these improvements have not been systematically analysed. Because ICE is used in different stages and in different ways throughout the procedure, it would be valuable to have a better understanding of the relationship between improvements in outcomes and the specific timing and methodology of how ICE is used. In addition, the site, mechanism, and underlying arrhythmic substrate contribute to study outcomes. It would be of interest to understand how ICE potentially impacts study outcomes among arrythmia types differently.
Although it is likely that improved outcomes with ICE will result in economic savings, conclusions regarding the cost-efficiency of ICE in ablation procedures could not be drawn from these studies. Further investigation, including cost-efficiency analyses, are necessary to validate and quantify the economic benefit associated with the use of ICE in ablation procedures. Furthermore, the incremental value of adopting ICE in simple procedures (e.g., supraventricular tachycardia and right ventricular outflow tract tachycardia) may be limited. Studies are also needed to understand how ICE might impact duration of these procedures.
CONCLUSION
In addition to previously established benefits associated with the use of ICE technology, these new publications strengthen the entirety of the body of evidence supporting the use of ICE in AF and VT ablation. Collectively, these studies indicate that the integration of ICE into ablation workflows significantly improves outcomes. These outcomes include reduced radiation exposure for patients and healthcare professionals, improved procedure efficiency, lowered incidence of complications, and reduced need for readmission or repeat ablations. Furthermore, it is anticipated that as ICE technology continues to develop and we see further advances in resolution and in 3D or four-dimensional image acquisition, the clinical utility of ICE will continue to expand.








