Interview Summary
The emerging class of agents targeting factor XI/XIa offers the paradigm-shifting possibility of ‘haemostasis-sparing anticoagulation’: protection from stroke and other thromboembolic events with a benign bleeding profile. With three investigational agents now into late-phase development, two presentations delivered at the European Society of Cardiology (ESC) Congress 2024, held in London, UK, have added to our knowledge of this innovative and diverse class. The first of these presentations shared the full data from the OCEANIC-AF Phase III trial, in which the small molecule factor XIa inhibitor asundexian was compared with the factor Xa inhibitor apixaban for stroke prevention in atrial fibrillation, and reviewed the probable reasons for the failure of this trial to meet its efficacy endpoint.
The second presentation was a secondary analysis from the AZALEA-TIMI 71 Phase II trial, in which the safety of the monoclonal antibody factor XI inhibitor abelacimab was compared with that of the factor Xa inhibitor rivaroxaban in patients with atrial fibrillation undergoing invasive procedures.
This article is based on a post-ESC interview with Jeffrey I. Weitz, Professor of Medicine and Biochemistry and Biomedical Sciences at McMaster University, Canada; Canada Research Chair (Tier 1) in Thrombosis and the Heart and Stroke Foundation; J.F. Mustard Chair in Cardiovascular Research; Executive Director of the Thrombosis and Atherosclerosis Research Institute (TaARI), in Hamilton, Canada; and Secretary General of the International Society on Thrombosis and Haemostasis (ISTH). It assesses the current status and future prospects of the factor XI/XIa inhibitor class in light of these recent developments.
INTRODUCTION
At the ESC Congress 2024 in London, Principal Investigator Manesh Patel from Duke University Medical Center, Durham, North Carolina, USA, reviewed the outcomes of the OCEANIC-AF Phase III trial, in which 14,830 patients with atrial fibrillation (AF) at high risk of stroke were randomised 1:1 to receive the small molecule factor XIa inhibitor asundexian, 50 mg once daily, or apixaban, 5 mg or 2.5 mg twice daily.1 The primary objective was to demonstrate whether asundexian was at least non-inferior in terms of efficacy, and superior in terms of safety, compared to apixaban.1 After a median follow-up of 160 days, asundexian 50 mg had succeeded in demonstrating superior safety to apixaban, but had failed to show non-inferior efficacy (Table 1).
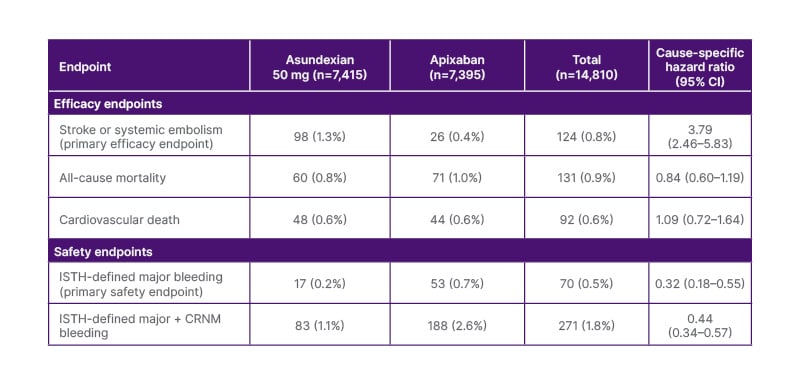
Table 1: Efficacy and safety outcomes of OCEANIC-AF.
Adapted from Piccini JP et al. 2024.1
CRNM: clinically-relevant non-major; ISTH: International Society on Thrombosis and Haemostasis.
As a result, in November 2023, the independent data monitoring committee (IDMC) recommended early termination of the trial.1 Patel reflected on the failure of OCEANIC-AF to achieve its efficacy endpoint and the implications for the rest
of the factor XI/XIa inhibitor class.
The following day, Siddharth Patel, from the TIMI Study Group and Brigham and Women’s Hospital in Boston, Massachusetts, USA, presented a secondary analysis from the Phase II AZALEA-TIMI 71 trial, comparing two doses of the long-acting monoclonal antibody factor XI inhibitor abelacimab with rivaroxaban in patients with AF.2 This analysis focused on trial participants undergoing invasive procedures, a common scenario in this patient population.3
In the trial, 1,287 patients were followed for a median of 2.1 years, and 920 invasive procedures were performed (34% in the abelacimab arms and 36% in the rivaroxaban arm). For procedures with a low or intermediate risk of bleeding (as defined by the 2017 American College of Cardiology [ACC] Periprocedural Management Expert Pathway),4 continuation of abelacimab was encouraged; for elective procedures associated with a high risk of bleeding, interruption was advised, while for non-elective procedures, the use of an antifibrinolytic plus low-dose recombinant factor VIIa was recommended.2 For patients on rivaroxaban, standard care was followed, typically involving interruption 24–48 hours before the invasive procedure. Most patients underwent low-risk procedures, and approximately one in four of the procedures were non-elective. Notably, 56% of procedures occurred within 30 days of the last monthly dose of abelacimab, when factor XI was still substantially inhibited, without interruption of the medication.2 In total, 0.8% of patients in the abelacimab arm versus 1.4% of patients in the rivaroxaban arm experienced a procedure-related major/clinically relevant non-major bleed, suggesting that, even though it is long-acting, routine interruption of abelacimab may not be necessary for many elective procedures.2 This article is based on an interview with Weitz, who was one of the AZALEA-TIMI 71 investigators, conducted soon after the ESC Congress 2024.
WHY IS THERE A NEED FOR NEW ANTICOAGULANTS?
Ever since the dawn of modern anticoagulation, Weitz observed, the agents available to us have collectively been seen as “a double-edged sword”, where protection from thromboembolism always goes hand-in-hand with a seemingly inescapable risk of bleeding. Compelled to walk a tightrope each time they prescribe an anticoagulant, Weitz added that clinicians are also faced with the uncomfortable truth that the patients who most need long-term anticoagulation, such as those with AF at risk of stroke, are also those for whom bleeding is particularly hazardous, since they are typically elderly, frail, and comorbid.5 Although the direct oral anticoagulants (DOAC) represented a major advance over warfarin and other vitamin K antagonists, there remains an annual 12% rate of major and clinically relevant non-major bleeding in patients with AF receiving oral anticoagulation.6 This risk, and the fear of it, largely explains why at least 40% of the AF population receives no anticoagulation at all,7-10 and why many of the remainder receive inappropriately low doses, which end up compromising efficacy without increasing safety.7, 11-13
In addition, patients with AF who do receive anticoagulants often skip doses or prematurely discontinue their medication,14,15 again often due to bleeding concerns. Weitz added that, in some cases, often without the prescriber’s knowledge, poor adherence or persistence may be driven by what we’ve traditionally termed ‘minor bleeding’, such as recurrent nosebleeds or bruising, which may ultimately impair quality of life or curtail lifestyles too much to be tolerated. This ‘patient-relevant bleeding’,16 Weitz reflected, probably contributes more than we’ve realised to the overall burden of bleeding experienced by patients on anticoagulation. So, while current anticoagulants are undoubtedly efficacious, prevailing safety concerns continue to be a key reason for the significant undertreatment of eligible individuals in the real world.5,8
WHY COULD TARGETING FACTOR XI/XIA OFFER AN ESCAPE ROUTE FROM THE INHERENT BLEEDING RISK OF CURRENT ANTICOAGULANTS?
Although pathological thrombosis and physiological haemostasis both depend on classical blood clotting protagonists like tissue factor, factor Xa, and thrombin, we’ve come to realise that the pathway leading to the formation of an occlusive intravascular thrombus is not the same as the pathway leading to the formation of a mostly extravascular haemostatic plug that helpfully seals a leak or injury in a blood vessel wall17 (Figure 1).
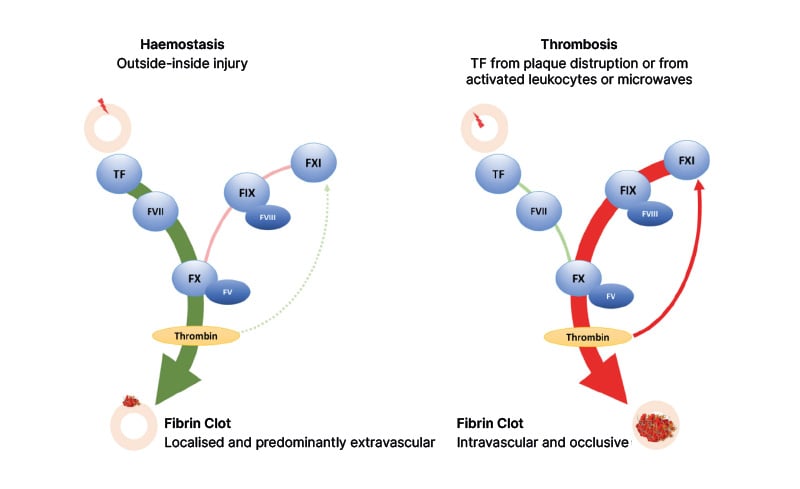
Figure 1: Pathways leading to haemostasis and thrombosis, showing the differential role of factor XI in each of them.
Adapted from Chan NC, Weitz JI. 2023.18
TF: tissue factor.
Downstream, the pathways are indistinguishable, so targeting downstream elements like factor Xa or thrombin (or their precursors factor X and prothrombin), as the DOACs and warfarin do, impacts both thrombosis and haemostasis, protecting patients from thromboembolic events but also exposing them to an increased risk of bleeding.17 However, if we look further upstream, the two pathways diverge. Here, attention has been focused on factor XI (and its activated form, factor XIa).
Weitz established that, for a long time, we’ve known that factor XI is non-essential for haemostasis, as shown by the fact that people with congenital factor XI deficiency have little or no risk of serious or spontaneous bleeding.19,20 We had assumed that, perhaps, factor XI was also non-essential for thrombosis, until relatively recent preclinical studies21 and epidemiological research22,23 suggested otherwise. We now know that, although factor XI is not needed to initiate the clotting process for either thrombosis or haemostasis, it plays a central role in the growth of thrombi.17 Once an intravascular clot begins to form, it covers up the damage to the vessel that triggered it in the first place, so for the clot to expand, thrombin has to feed back and activate factor XI.17 This feedback loop generates more factor Xa and more thrombin, ultimately promoting clot growth.17
In contrast, haemostasis is triggered by the large amounts of tissue factor in the haemostatic ‘envelope’ surrounding the site of injury to a vessel, resulting in such explosive thrombin generation that the feedback process involving factor XI is mostly unnecessary, Weitz explained, adding that “it’s rather like the in vitro test, the prothrombin time, where adding large amounts of thromboplastin (which is essentially tissue factor and calcium) to plasma promotes clot formation in just a few seconds”. Factor XI plays no role in this accelerated process.17 Thus, by pharmacologically targeting factor XI, the two pathways can be conceptually ‘uncoupled’. This raises the unprecedented possibility of ‘haemostasis-sparing’ anticoagulation,17, 24-27 which, Weitz said, could be truly transformative if it becomes a reality.
WHAT TYPES OF INVESTIGATIONAL AGENTS CURRENTLY COMPRISE THE EMERGING FACTOR XI/XIA INHIBITOR CLASS?
Unlike the factor Xa inhibitors (DOAC), the emerging class of investigational factor XI/XIa inhibitors is heterogeneous in many ways.24,25,27 Weitz explained that the oral small molecules asundexian and milvexian, taken daily and twice daily, respectively, bind reversibly to the active site of factor XIa (the activated form of factor XI), much as DOACs bind reversibly to the active site of factor Xa or thrombin. In contrast, the monoclonal antibody abelacimab, which is administered subcutaneously once a month for stroke prevention in AF, targets the zymogen (inactive form) of factor XI, preventing its activation to factor XIa, and also neutralises any existing factor XIa.28 These are the current frontrunners in the class. At an earlier stage of research and development, there are other investigational monoclonal antibodies with slightly different mechanisms of action. They also have novel strategies to reduce the synthesis of factor XI, including a first- and second-generation antisense oligonucleotide. Weitz categorises members of this expanding class as “those that inhibit factor XIa activity” and “those that prevent or attenuate factor XIa generation”, with some agents straddling both. The collective ‘magic’ of this class, Weitz said, is that they all, in some way, suppress the effect of factor XI, and thus all have the potential to uncouple haemostasis from thrombosis. However, they also have numerous differences, both mechanistic and pharmacological, that may be significant in real-world clinical contexts.29
WHAT EVIDENCE EXISTS SO FAR FOR THE EFFICACY OF THE CLASS, AND WHAT COULD EXPLAIN THE FAILURE OF THE OCEANIC-AF TRIAL WITH ASUNDEXIAN?
Weitz confirmed that the gold-standard first step in the clinical development of prospective new anticoagulants is testing them as prophylaxis for venous thromboembolism (VTE) after elective joint replacement surgery.5 This model exploits the high risk of postoperative deep vein thrombosis that can be efficiently detected via venography 10–12 days after the surgery. Four investigational factor XI/XIa inhibitors have been evaluated for VTE prophylaxis in patients undergoing elective total knee replacement: milvexian, abelacimab, osocimab (another investigational monoclonal antibody), and the first-generation antisense oligonucleotide, FXIRx. In randomised open-label Phase II studies, all showed superior efficacy to standard-of-care low molecular weight heparin enoxaparin.30-34 The reduction in VTE incidence with factor XI/XIa inhibitors compared to enoxaparin was 40–50% overall,34 and as high as 80% with the 150 mg dose of abelacimab.31 This reduction in VTE was associated with an overall 60% reduction in bleeding compared with enoxaparin.34 These findings comprise an encouraging sign of class efficacy, supporting the central hypothesis that suppressing the effects of factor XI is therapeutically beneficial and may uncouple thrombosis from haemostasis.
Importantly, in addition to providing proof-of-concept for efficacy, these joint replacement studies were also designed to find the most appropriate dose of each investigational agent to take forward to Phase III. It is notable, Weitz pointed out, that a Phase II study of this nature was never undertaken with asundexian, which raises questions over the selection of the 50 mg dose used in the Phase III OCEANIC-AF study.1 Weitz stated: “The failure of asundexian to show non-inferior efficacy to apixaban in this pivotal trial was, of course, disappointing, but I’m convinced the explanation comes down to the use of a dose that failed to inhibit factor XIa sufficiently. If 50 mg of asundexian really does produce 94–95% inhibition of factor XIa1, how could it fail in an efficacy study? I can understand why this outcome might have led some observers to question the whole concept of factor XI/XIa inhibition, but I think we have to step back and question how factor XIa inhibition by asundexian was measured.”
Weitz went on to confirm that there is no universal assay that enables us to determine, or compare, the level of inhibition of factor XI/XIa by the different investigational agents.5 The assay that was used for asundexian was a proprietary assay based on inhibition of endogenous factor XIa generation. Essentially, it involved inducing factor XIa generation in the patient’s plasma, and then measuring the residual factor XIa activity in the absence or presence of the drug.35 “We don’t know much about endogenous factor XIa generation, how this might vary from person to person, and how relevant it might be with an artificial activator of the pathway. Whatever the approach, it is imperative for any new assay to seek correlation with clinical outcomes before it can be seen as robust or dependable,” Weitz said.
The Phase II programme for asundexian in AF, acute myocardial infarction, and stroke was well powered for safety but not for efficacy,36 so provided no opportunity for correlation of the assay with efficacy outcomes. Instead of conducting a Phase II dose-finding study in the gold-standard orthopaedic surgery model, it appears, according to Weitz, that the dose of asundexian chosen for OCEANIC-AF may have simply been based on data with the FXIa inhibition assay. It is known that milvexian, the other investigational small molecule factor XIa inhibitor “is a little more potent against factor XIa than asundexian;37 it produces a little more prolongation of the activated partial thromboplastin time (aPTT), probably reflecting slightly greater potency and a little less protein binding, leaving more of the drug-free to act on factor XIa.” But, even if it is assumed that the FXIa inhibitory activity of asundexian is the same as that of milvexian, a 50 mg dose of milvexian (which was the dose chosen for asundexian in OCEANIC-AF) was shown in the AXIOMATIC-TKR study to have similar antithrombotic activity to enoxaparin.32 In Weitz’s view, it is not clear why a dose showing only comparable antithrombotic activity to a prophylactic dose of enoxaparin would be taken forward to Phase III for study in AF.
Weitz continued: “So that’s one clue that the dose of asundexian in OCEANIC-AF was too low. Another clue is that, with the 50 mg once daily dose of asundexian, the aPTT fell to 1.4 by the trough before the next dose, and yet the AXIOMATIC-TKR study with milvexian showed that the aPTT really needed to be kept around the two-fold prolongation mark throughout the treatment period to maintain superior efficacy to that of enoxaparin.32 So, I think everything points to the fact that the proprietary assay of factor XIa inhibition with asundexian was misleading.” Given that 50 mg of asundexian, a rather low dose, produced 94–95% factor XIa inhibition with this assay, it is also clear the assay is going to ‘max out’ with higher doses, Weitz observed. “It’s evidently very sensitive, but we don’t really know what it means, and none of the other factor XI/XIa inhibitor agents are using this assay for their dose-finding,” Weitz said. In response to Manesh Patel’s speculation that total suppression of factor XIa activity may be needed for adequate efficacy in this indication,38 Weitz commented that “this could be true, but everything depends on how factor XIa inhibition is measured. With a different assay, pre-correlated with clinical outcomes, 94–95% inhibition may well have been sufficient for non-inferiority compared with apixaban.”
COULD INTRA-CLASS DIFFERENCES IN MECHANISMS OF ACTION ALSO BE CLINICALLY RELEVANT?
The investigational agents within the emerging class of factor XI/XIa inhibitors have diverse mechanisms of action (Figure 2).
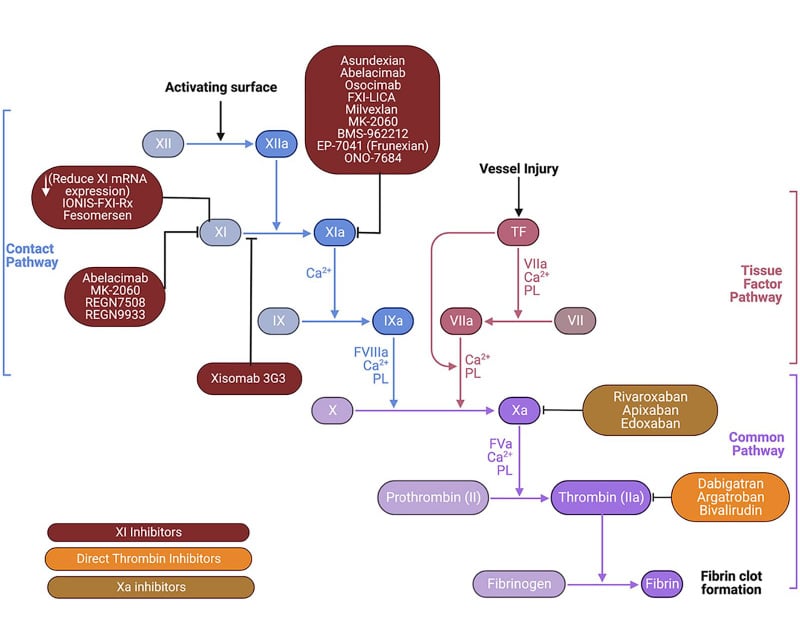
Figure 2: Mechanisms of action of factor XI/XI inhibitors compared to direct oral anticoagulants.
Adapted from Prakash S et al. 2024.39
PL: phospholipids TF: tissue factor.
As mentioned, the small molecules asundexian and milvexian bind reversibly to the active (catalytic) site of factor XIa to block its activity.24 Since these inhibitors require factor XIa to be formed before they can act, and then have to compete with its natural substrate, factor IX, for the catalytic site, it is theoretically possible, Weitz speculated, that some factor XIa may evade inhibition, resulting in less than optimal anticoagulant efficacy. In contrast, the monoclonal antibody abelacimab targets the factor XI zymogen and locks it in its inactive form, preventing generation of factor XIa,24 as well as neutralising any existing factor XIa.28 This mechanism of action more closely simulates natural factor XI deficiency, which is known from epidemiological research to confer protection from thromboembolic disease.19 Weitz observed that, currently, there is no hard evidence for the advantages of one mechanism of action over another, and as with DOACs, there are no head-to-head trials of factor XI/XIa inhibitors. However, given the highly thrombogenic activity of factor XIa, Weitz believes that “it is conceivable that the greater the suppression, the greater the efficacy. And if this is true, safety becomes paramount.”
TURNING TO SAFETY, HOW SURPRISED WERE YOU BY THE RESULTS OF AZALEA-TIMI 71?
In Weitz’ view, there was already a clue from the four Phase II total knee arthroplasty studies (conducted with milvexian, abelacimab, osocimab, and FXIRx) that pharmacological factor XI/XIa inhibition causes less bleeding than current approaches to anticoagulation.30-34 For this reason, Weitz explained, “I was hopeful that AZALEA-TIMI 71, which aimed to compare the bleeding profile of abelacimab, dosed once monthly, with that of rivaroxaban in an AF population,40 would be confirmatory. When the superior safety of abelacimab was indeed demonstrated in AZALEA-TIMI 71, which had a median follow-up of 21 months,40,41 the data exceeded my expectations, especially since abelacimab is a potent drug that achieves almost total (approximately 99%) suppression of factor XIa generation throughout its long dosing interval.”40,41 What impressed Weitz most of all was the lack of gastrointestinal bleeding with abelacimab.40,41 The main weakness of the DOACs is their association with 25% more gastrointestinal bleeding than warfarin,5,42 probably because DOACs are active drugs in the gut.43 “I found it fascinating that the use of once-monthly abelacimab in place of a DOAC could simply erase one of the most common bleeding problems these patients experience,” Weitz said.
WHAT IS YOUR REACTION TO THE AZALEA-TIMI 71 PERI-PROCEDURAL DATA?
Weitz considers these peri-procedural data to be very important because elderly patients with AF receiving anticoagulation often have to undergo invasive procedures of various kinds.3 With DOACs, best practice is to suspend anticoagulation before elective procedures, but it was important to know how necessary this is for factor XI/XI inhibitors, and most particularly for abelacimab, given it is a long-acting agent with a 30-day half-life.44 The study showed that, for procedures associated with a low-to-moderate risk of bleeding, it was safe to carry these out within 30 days of the last dose of abelacimab.2 Further data are needed for patients undergoing high bleeding risk procedures and/or non-elective procedures within the first 30 days post-dose, of whom there were very few in this study. However, Weitz believes the data so far are encouraging and suggests that routine interruption of anticoagulation may not be necessary with abelacimab.2
HOW MIGHT THE PHARMACOLOGICAL DIFFERENCES WITHIN THE FACTOR XI/XI CLASS AFFECT THEIR USE IN THE REAL WORLD?
The heterogeneity of this class means that we are looking at different modes of administration and different dosing frequencies, Weitz explained (Table 2).
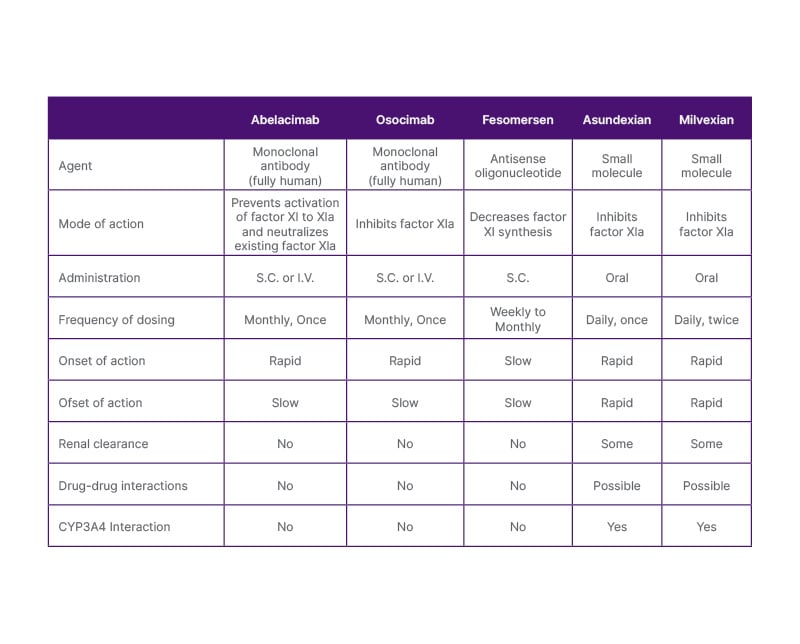
Table 2: Mechanistic and pharmacological differences between factor XI/XIa inhibitors.
Adapted from Fredenburgh JC, Weitz JI,29 2021.
I.V.: intravenous; S.C.: subcutaneous.
Less frequent dosing, such as with once-monthly abelacimab, may help circumvent the notorious problem of poor adherence that is typically seen with daily or twice-daily dosed oral medications in preventative settings. Other real-world concerns with DOACs include the need for dose adjustment in patients with renal or hepatic impairment and the potential for drug-drug interactions (DDI),45 a particular worry in patients with cancer. When using an antibody rather than a small molecule, renal clearance is not an issue because antibodies are not cleared through the kidneys. In addition, antibodies essentially dial out DDI concerns.46 Although any individual DDI might be relatively minor, the interplay of numerous concomitant drugs may have unknown and unforeseen consequences, which we cannot monitor. So, in Weitz’s view, avoiding all of that could be a huge advantage.
IN THE WAKE OF OCEANIC-AF, WHAT REASONS ARE THERE FOR CONTINUED OPTIMISM ABOUT FACTOR XI/XIA INHIBITION?
Weitz believes there are many reasons for optimism about factor XI/XIa inhibition. When the early termination of OCEANIC-AF was announced, this naturally created heightened concern about other ongoing trials of factor XI/XIa inhibitors. As a result, their IDMCs have been scrutinising things very closely, and none of them have been halted or suspended. The Phase III LIBREXIA-AF trial with milvexian (NCT05757869) 47 is now at a similar stage to where OCEANIC-AF was when it was terminated.
Asundexian’s secondary stroke prevention trial, OCEANIC-Stroke (NCT05686070),48 is also ongoing. This study is using the same 50 mg dose as in OCEANIC-AF, but this time compared with placebo, on top of single or dual antiplatelet therapy, a very different scenario. It is noteworthy that this trial, as well as milvexian’s LIBREXIA-STROKE (NCT05702034)49 and LIBREXIA-ACS (NCT05754957),50 are all capitalising on the predicted superior safety of factor XI/XIa inhibition by using them in dual pathway inhibition models as a safer platform for antiplatelet therapy. The LILAC-TIMI 76 trial with abelacimab (NCT05712200)51 is going even further, capitalising on abelacimab’s benign bleeding profile by enrolling patients whose pre-existing high risk of bleeding has made them (in the opinion of their own physicians) clinically ineligible for current oral anticoagulant options.
The progress of the ASTER (NCT05171049)52 and MAGNOLIA (NCT05171075)53 studies with abelacimab in cancer-associated thrombosis is also encouraging. Weitz pointed out that no one could really predict how well factor XI/XIa inhibition might work in people who already have thrombosis (where factor XI has already been activated to form factor XIa, with consequent generation of downstream clotting factors), and added that “if there’s any group of patients who are at particularly high risk for recurrent thrombosis, it is patients with cancer”. Yet ASTER and MAGNOLIA seem to be on track as far as their IDMCs are concerned, with enrolment at an advanced stage. The other big reason for optimism, according to Weitz, is the impressive safety record of factor XI/XIa inhibitors. In particular, the AZALEA-TIMI 71 trial with abelacimab has shown convincingly that factor XI inhibition is indeed haemostasis-sparing.2,40,41
If the Phase III efficacy trials we are waiting for provide the evidence we are seeking, Weitz said, then the proven benign bleeding profile of this class “should empower us to strive for maximum protection for our patients, essentially removing the safety ‘handcuffs’ we’ve all been obliged to wear for so long”.







