Abstract
Cardiotoxic effects in patients with breast cancer may present as asymptomatic left ventricular (LV) dysfunction or symptomatic LV decline, which can progress to overt heart failure (HF). Trastuzumab is a monoclonal antibody against human epidermal growth factor receptor (HER)2 and is a recommended targeted treatment for patients with overexpression of this receptor. However, the use of trastuzumab is associated with cardiotoxicity, manifested as LV dysfunction or HF. This review addresses the key issues related to individualised cardioprotection and surveillance, especially in elderly patients with HER2-positive breast cancer, based on the current cardio-oncology literature. Cardiac imaging techniques (e.g., echocardiography or multiple-gated acquisition scan) and biomarkers (e.g., cardiac troponins) that play a crucial role in the detection and monitoring of cardiotoxicity related to systemic therapies for breast cancer are briefly described. This review presents cardioprotective approaches, including interruption or termination of trastuzumab therapy, and treatment with an angiotensin-converting enzyme inhibitor, angiotensin-receptor blocker, or beta-blocker, which have been recommended for the reduction of cardiac adverse effects. Since the data relevant to cardiotoxicity of trastuzumab among real-world older women with breast cancer and cardiovascular diseases are still limited, this article focusses on improvements to the cardiac safety of trastuzumab-based regimens. In particular, this review emphasises the importance of intense surveillance in the elderly female population.
INTRODUCTION
Due to recent advances in antineoplastic treatment for breast cancer, cancer-related mortality has substantially decreased. However, several systemic therapies have contributed to cardiac complications that adversely influence patient outcomes.1 In patients with human epidermal growth factor receptor (HER)2-positive breast cancer, treatment with trastuzumab and anthracyclines has been associated with short and long-term cardiotoxicity. Therefore, reducing the negative impact of these antineoplastic agents on the cardiac condition, via early detection, immediate treatment, and regular monitoring, is a key objective in breast cancer management, particularly among elderly patients who often present with pre-existing cardiovascular disease (CVD). In a real-world hospital setting, female patients aged ≥65 years diagnosed with breast cancer have been considered to be candidates for systemic therapy if the expected advantages of such a therapy would outweigh the risks (e.g., cardiac adverse effects).1 It has been estimated that approximately 15–25% of breast cancers consist of HER2-positive tumours.1 Trastuzumab (Herceptin®; Roche Holding AG, Basel, Switzerland) is a humanised monoclonal antibody that binds to the extracellular domain of HER2 and targets the HER2 signalling pathway.1,2 Herceptin is a targeted anticancer therapy that was approved for clinical use in 1998 and can be used concurrently or sequentially with systemic chemotherapy (CHT), leading to the improvement of disease-free survival and overall survival of women with HER2-positive breast cancer, both in early and metastatic stages.2
However, trastuzumab can also cause treatment-induced cardiotoxicity, which manifests mostly as a decline in left ventricular ejection fraction (LVEF) (often asymptomatic) or abnormal cardiac function that may progress to overt heart failure (HF).2 For instance, according to some clinical studies, cardiotoxic side effects occurred in up to 27% of patients who received trastuzumab concurrently with anthracyclines, which are highly cardiotoxic agents, but in <13% of those who received trastuzumab with paclitaxel.3 Cardiotoxicity related to trastuzumab and anthracyclines, as well as to other anticancer therapies, remains a serious concern.4 Therefore, the key goal is to improve the cardiac safety of trastuzumab. This may require a more intense surveillance in elderly patients compared with their younger counterparts. For this reason, in older women (who often present with multiple morbidities and have a greater incidence of cardiomyopathy and HF), routine surveillance should be optimised (e.g., via a vigilant diagnostic approach and the implementation of more aggressive cardioprotective measures, including pharmacologic and non-pharmacologic modalities, such as dietary, educational, or lifestyle-related strategies).1,4 This is consistent with recent findings from the HERA trial,5 which have shown that cardiotoxicity events associated with trastuzumab were less severe when patients were screened in a very strict manner for pre-existing cardiac conditions.5 Furthermore, the final analysis of the HERA trial, including 11 years of follow-up of trastuzumab after adjuvant CHT in patients with HER2-positive early breast cancer, revealed that 1 year of adjuvant trastuzumab treatment after CHT significantly improved long-term disease-free survival, while 2 years of trastuzumab treatment had no additional benefit.5
MECHANISMS UNDERLYING TRASTUZUMAB-INDUCED CARDIOTOXICITY
The mechanism underlying trastuzumab-induced cardiotoxicity remains unclear. Oxidative stress and the production of highly toxic free radical molecules are thought to be the main culprits for the induction of myocyte injury. In addition, it has been suggested that blockage of HER2 receptors can contribute to trastuzumab-induced cardiotoxicity because HER2 receptors are expressed on cardiac myocytes and play a crucial role in embryonic cardiac development, as well as growth and repair of the heart muscle in adult life.4 Cardiotoxicity due to trastuzumab is mostly reversible and not dose dependent. The effects of trastuzumab are characterised as type 2 CHT-related cardiac dysfunction. In contrast, drugs commonly used in therapy with anthracyclines, such as doxorubicin, represent a type 1 CHT-related cardiac dysfunction, manifested as an irreversible, dose-dependent myocardial damage.4 It should be highlighted that the highest risk of cardiotoxicity induced by trastuzumab occurs in cases of concurrent treatment with anthracycline-based regimens in patients presenting with advanced age, Type 2 diabetes mellitus, decreased glomerular filtration rate, low baseline LVEF, arterial hypertension, or pre-existing CVD.2-4
Trastuzumab-induced cardiotoxicity can negatively interfere with a recommended course of anticancer treatment. Under these circumstances, it has been recommended to monitor LVEF before starting trastuzumab therapy (at baseline), every 3 months during the treatment course, and then 6 months after treatment completion.1 While the risk of overt HF during treatment with trastuzumab is rather low (1–4% of patients), asymptomatic decline of LVEF is much more frequent (7–19% of patients) according to the results of recent clinical trials.2,5 It should be underscored that in the hospital setting the rates of cardiac side effects are even higher, especially in elderly women with CVD or risk factors for such cardiovascular comorbidities.1,3,6 In daily clinical practice, this discrepancy can be even more aggravated because such patients have often been excluded from participation in randomised clinical trials (RCT).
Moreover, it should be underscored that the cardiotoxicity secondary to trastuzumab exposure is dose-independent, meaning that it can unpredictably escalate to HF. However, if promptly detected and adequately treated, this condition is usually reversible.7 Additionally, it has been found that after the resolution of trastuzumab-induced cardiac adverse effects, trastuzumab therapy can be restarted following the implementation of a standard therapy for HF under close supervision of a consulting cardiologist.7 In addition, since a commonly used CHT regimen containing anthracyclines predisposes a patient to trastuzumab-induced cardiotoxicity, the incidence of adverse cardiac effects is highest when anthracyclines and trastuzumab are administered concurrently. In contrast, cardiotoxicity declines as the time period between anthracycline and trastuzumab administration increases and, when trastuzumab is used in sequence (after completing a course of CHT with anthracyclines).8
CARDIOTOXICITY OF TRASTUZUMAB IN ELDERLY PATIENTS: IMPORTANT CONSIDERATIONS
In general, in the older population (>65 years of age), biological characteristics of breast cancer, including hormone receptor (HR) positivity, low mitotic rate, low nuclear grade, rare p53 gene mutations, and relatively infrequent overexpression of epidermal growth factor receptor or HER2, are often more favourable than in younger patients.9,10 However, in spite of that, mortality rate remains higher in older versus younger women,11 and this can be due to the different behaviour of tumours in elderly patients or a possible risk of death from comorbid diseases.9,10
Trastuzumab therapy should be considered for elderly patients with early-stage breast cancer (e.g., HER2-positive and HR-negative or HER2-positive and HR-positive with lymph node invasion) and with more advanced malignancy, but the cardiotoxicity of the treatment must always be paramount.12-14 According to a study that assessed standard CHT with or without trastuzumab in patients with metastatic breast cancer, it was revealed that cardiac dysfunction (New York Heart Association [NYHA] Class III or IV) was recorded in 16% of patients using anthracycline, cyclophosphamide, and trastuzumab, compared to 3% of those who were treated only with anthracycline and cyclophosphamide. In addition, in this trial, NYHA Class III or IV was reported in 2% of female patients receiving paclitaxel and trastuzumab, compared to 1% of those who were treated with paclitaxel alone.15
In the landmark study NSABP B-31,16 which compared anthracycline and cyclophosphamide, followed by paclitaxel with anthracycline and cyclophosphamide, followed by trastuzumab and paclitaxel, the participants had HER2- positive and node-positive breast cancer, and normal post-CHT LVEF (as per multiple-gated acquisition [MUGA] scan evaluation). In this study population, the incidence of adverse cardiac events was 4.1% in the trastuzumab group compared to 0.8% in the control group after 3 years, and 4.0% versus 1.3%, respectively, at 7 years of follow-up.16 Similarly, in the NCCTG N9831 trial,17 which also evaluated the efficacy and safety of trastuzumab in addition to paclitaxel after anthracycline (doxorubicin) and cyclophosphamide CHT, the rates of HF in the trastuzumab versus control groups were 3.8% versus 1.3%, and 2.3% versus 0.9%, respectively.
According to the results of a meta-analysis of eight RCT, which addressed the use of trastuzumab for non-metastatic or locally advanced breast cancer, the incidence of symptomatic HF ranged from 0.8–14.2% among trastuzumab-treated patients, compared to 0.2–4.1% in the control group.18 Based on data from a European cancer registry, in which 32.6% of patients were ≥60 years of age, the cumulative risk of trastuzumab-related cardiotoxicity for the females >70 years old compared with the younger patients was 6.4% versus 1.3% after 1 year, 9.8% versus 2.0% after 2 years, and 9.8% versus 2.2% after 3 years of treatment, respectively.19 This is concurrent with the USA cancer registry that included trastuzumab-treated HER2-positive metastatic breast cancer patients, in which the incidence of HF for women >75 years old was 3.2%, between 65 and 74 years was 1.9%, and <65 years of age was 1.5%.20 Convergent with these results, in a group of German females who were taking trastuzumab in an adjuvant setting, the frequency of decline in LVEF or an overt HF was 3.7% in the subgroup of women <65 years of age, 3.9% in the patients between 65 and 69 years of age, and 5.7% in the women ≥70 years of age.21
In general, several studies have revealed that trastuzumab, with or without anthracyclines, contributed to a risk of acquiring HF that can be estimated as two-fold higher than that occurring in patients who did not receive trastuzumab therapy.22 It should be underscored that the hospital-based studies take into consideration many elderly patients, treated daily in clinical practice, who otherwise would have been excluded from RCT. Since the mortality rate 5 years after diagnosis of HF is about 50% in patients >65 years, a close surveillance of early HF symptoms and LV function in this elderly breast cancer population treated with trastuzumab is critically important.22
ADVANTAGES OF USING CARDIAC BIOMARKERS FOR EARLY IDENTIFICATION OF MYOCARDIAL INJURY IN PATIENTS WITH HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2-POSITIVE BREAST CANCER TREATED WITH TRASTUZUMAB OR ANTHRACYCLINES
Recently, cardiac biomarkers have been investigated for their potential as early detectors of anticancer therapy-induced cardiotoxicity. While the role of biomarkers in monitoring anthracycline toxicity is generally well understood, substantial uncertainty remains regarding their role in monitoring targeted HER2 anticancer treatments. Cardiac troponins and N-terminal pro-B-type natriuretic peptide (BNP) are gaining interest in monitoring cardiac toxicity with trastuzumab.23 There is some variability among biomarker studies, especially regarding the sensitivity or specificity of the biomarker assays and reference ranges for normal versus abnormal results. However, despite these issues, there is an agreement that troponin I predicts early, but not late, cardiac events induced following trastuzumab administration after anticancer regimens that contain anthracyclines. Moreover, troponin increases may illustrate cardiac damage due to anthracyclines rather than trastuzumab-induced myocardial injury.23 Therefore, troponin levels can serve as biomarkers of susceptibility to cardiotoxicity during the early period of trastuzumab therapy, especially in patients with HER2-positive breast cancer who have recently received CHT regimens with anthracyclines.23 In addition, elevation of the troponin levels would enable an immediate introduction of preventive measures before the onset of functional LVEF deterioration. For this reason, cardiac troponin I (cTnI) and T (cTnT) can be used as biomarkers of myocardial injury in a population with HER2-positive breast cancer,23 as illustrated in recent research trials. For instance, in a study by Cardinale et al.,23 the plasma levels of cTnI patients with different types of neoplasms who received high doses of CHT were measured after each CHT cycle. The study participants were divided into troponin positive (cTnI+) and troponin negative (cTnI-) subgroups. In the cTnI- subgroup, there was a transient decline of LVEF post CHT (with a nadir after 3 months from baseline), followed by a subsequent recovery. In contrast, participants in the cTnI+ subgroup had bigger declines in their LVEF, which persisted for up to 7 months of monitoring.23
The impact of persistent elevations of cTnI was assessed in another trial, and the results showed that the patients who received high-dose CHT and had persistent increases in cTnI had a higher incidence of cardiotoxicity (85%) compared to the patients with only transient increases of cTnI (37%) or to patients that had no elevation of cTnI (1%).24 It should be noted that BNP represents a biomarker of volume overload, and persistent N-terminal pro-BNP increase after administration of CHT has been correlated with a significant drop of LVEF.25 However, further studies are required to reveal whether N-terminal pro-BNP may serve as a predictive marker of trastuzumab and/or CHT-induced LV dysfunction. The role of high-sensitivity C-reactive protein is still controversial; however, it has been reported that high-sensitivity C-reactive protein can be a valid biomarker of trastuzumab-induced cardiotoxicity.26 Due to the heterogeneity of the examined populations, these findings have to be considered with caution. Furthermore, more research trials in this area are certainly merited.
CARDIAC IMAGING FOR DETECTION AND MONITORING OF TRASTUZUMAB-INDUCED CARDIOTOXICITY
Current techniques for detecting cardiotoxicity predominantly rely on assessment of LVEF via echocardiography (ECHO) or MUGA scan. However, in some patients, by the time a decline in LVEF is detected, there has already been subclinical myocardial injury. Therefore, there is a necessity to design and implement biomarkers that will facilitate the early detection of myocardial damage.22,27,28
Unquestionably, cardiac function should be monitored during trastuzumab treatment, and a decrease in LVEF of ≥10–<55% (often asymptomatic) has been found to be clinically relevant.27 LVEF has most usually been measured via conventional two-dimensional (2D) transthoracic ECHO or radionuclide ventriculography (MUGA scan). However, these methods lack precision and have been reported to miss some subclinical cardiac abnormalities.27
On the other hand, prompt detection of trastuzumab-induced cardiotoxicity followed by immediate administration of cardioprotective medications prior to any further decrease in cardiac function or appearance of HF symptoms is critical for both the patient and the treating team (especially for a cardiologist and an oncologist).
Regarding the cardiac imaging tools, some modern ECHO techniques, as well as cardiac MRI, have shown promise for more accurate diagnosis of LV dysfunction, which is relevant to trastuzumab. For instance, three-dimensional (3D) ECHO was indicated as a better tool than 2D ECHO for precise measurements of LVEF.29 According to the recommendations of the European Association of Cardiovascular Imaging (EACVI),30 standard parasternal long axis and apical view recordings should be carried out in the end-expiratory phase, with the patients in the supine left lateral position. An average of three cycle recordings should be used for standard measurements of cardiac dimensions and function. Biplanar apical 2 and 4-chamber views should be used to measure left atrial volume. All measurements need to be performed in end systole. In addition, specific 3D loops recorded from the apical view by storing four heart cycles allows for the analysis of LV volumes.30
AN INTEGRATIVE DIAGNOSTIC APPROACH: A COMBINATION OF CARDIAC IMAGING AND BIOMARKERS IN SURVEILLANCE OF CARDIOTOXIC EFFECTS RELATED TO TRASTUZUMAB AND ANTHRACYCLINES
According to the Society for Cardiovascular Magnetic Resonance (SCMR) guidelines, in cardiac MRI analysis, which can be performed using dedicated software, the epicardial and endocardial contours can be traced in end diastole and end systole. Simultaneously, calculation of ventricular volumes, LVEF percentage, and LV mass are being performed. Moreover, 3D ECHO was demonstrated to be more reproducible over a long period of monitoring and more consistent between different cardiologists performing serial ECHO exams, since both intra-observer and inter-observer variability for LVEF are minimised.29,30
A recent study by Kang et al.31 investigated whether changes of myocardial strain and high-sensitive cTnT may predict future cardiac dysfunction among patients who were previously exposed to anthracycline (e.g., epirubicin). In this study, the patients were examined using 2D speckle tracking ECHO (2D-STE) at baseline and then again during follow-up. Calculated global longitudinal strain (GLS), global circumferential strain (GCS), and global radial strain (GRS) were performed using 2D-STE, while LVEF was evaluated by real-time 3D ECHO.31 In this trial, in which cardiotoxicity criteria included a decrease of the LVEF of ≥5–<55% with symptomatic HF or an asymptomatic LVEF decline of ≥10–55%, the decrease of GLS was an independent predictor of cardiotoxicity.31 In addition, GLS combined with cTnT could provide a reliable way to predict cardiac dysfunction in patients receiving anthracycline-based chemotherapy.31 Similarly, the clinical value of 2D-STE, combined with high-sensitive cTnT in early detection of the cardiotoxicity induced by anthracyclines and trastuzumab, was also investigated in a study by Ho et al.32 The long-term effects of standard CHT on myocardial function in asymptomatic breast cancer survivors were examined using 2D-STE.According to this trial, some subclinical systolic and diastolic myocardial dysfunctions were reported in asymptomatic survivors of breast cancer (up to 6 years post standard CHT).32
In summary, an integrative approach that uses both cardiac imaging and biomarkers to identify cardiotoxicity of anticancer medications has beenhelpful regarding trastuzumab and anthracycline CHT, but this methodology requires further study in various therapeutic contexts, especially in theelderly patient population.28
AN OPPORTUNITY FOR PREVENTION AND TREATMENT OF TRASTUZUMAB AND ANTHRACYCLINE-INDUCED CARDIOTOXICITY
Trastuzumab-induced cardiotoxicity is reversible and discontinuation of this agent may be needed in up to 19% of patients.33 Some studies report that angiotensin-converting enzyme inhibitors (ACEI) and beta-blockers (BB) have protective effects in CHT-induced cardiotoxicity. Cardinale et al.34 reported prevention of AC-induced cardiomyopathy using the ACEI enalapril. Likewise, Kalay et al.35 reported a beneficial effect of carvedilol (a BB) use for prevention of cardiotoxicity. The MANTICORE trial was designed to assess the effectiveness of an ACEI (perindopril) and BB (bisoprolol) in primary prevention of trastuzumab-induced cardiotoxicity (Table 1).36,37 Results of the MANTICORE trial have revealed that bisoprolol and perindopril did not prevent LV remodeling; however, the two drugs had beneficial effects on LVEF among patients with HER2-positive, invasive, early breast cancer treated with trastuzumab.37 Furthermore, the PRADA trial showed that the angiotensin-receptor blocker candesartan, but not the BB metoprolol, resulted in less early LVEF deterioration compared to placebo during anthracycline-containing CHT with or without trastuzumab and radiation therapy (Table 1).38,39 Moreover, in the PRADA study, an anticancer treatment was associated with a modest, short-term decline in LV function. However, a long-term assessment is necessary and, if sustained (meaning that the long-term effect of early angiotensin inhibition can be confirmed), preventative therapy with angiotensin-receptor blocker may be recommended as a standard of care. Therefore, in patients treated for early, HER2-positive breast cancer, concomitant therapy with candesartan may provide protection against early decline in LV function.39 There is a growing need to adequately assess cardiac safety during anticancer therapy, especially among elderly patients and cancer survivors regardless of their age. At this point, cardio-oncology, as an emerging medical speciality, requires organisational and educational training support, research infrastructure, and well equipped clinical facilities. This is in accordance with an international consensus, under the auspices of the International CardioOncology Society and the Canadian Cardiac Oncology Network (CCON); both regional and international collaborative efforts in clinical research and practice are essential for real progress in this new interdisciplinary speciality.40
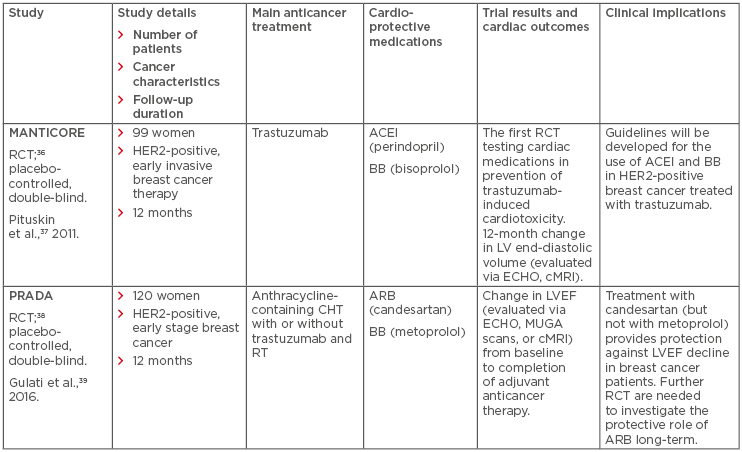
Table 1: Pharmacotherapy for prevention of trastuzumab-induced cardiotoxicity in patients with human epidermal growth factor receptor 2-positive breast cancer based on two major randomised controlled trials.
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin-receptor blocker; BB: beta-blocker; cMRI: cardiac magnetic resonance imaging; CHT: chemotherapy; ECHO: echocardiography; HER2: human epidermal growth factor receptor 2; LV: left ventricle; LVEF: left ventricular ejection fraction; MUGA: multiple-gated acquisition scan; RT: radiotherapy; RCT: randomised controlled trial.
In general, for elderly patients, the treatment teams should agree that numerical age is not so important for treatment decision-making. In contrast, individual cardiovascular risk factors, CVD or other comorbidities, overall life expectancy, quality of life, and patient preferences should play a main role in the multidisciplinary management of such patients.41,42
CONCLUSION
Targeted anticancer therapy, such as treatment with trastuzumab, has shown a 50% reduction in cancer recurrence rates and >30% improvement in survival outcomes of patients with HER2-overexpressing breast cancer. However, trastuzumab has contributed to various cardiotoxic effects (e.g., asymptomatic LVEF decline or overt HF onset), especially among elderly patients. In a real-world hospital setting, an appropriate assessment of patients by a multidisciplinary team prior to the initiation of trastuzumab is necessary to minimise the risk of potential cardiac adverse effects. Furthermore, using cardiac biomarkers (e.g., troponin I) for screening can be helpful for the early detection of myocardial injury among the most vulnerable patients. Both physicians and patients should keep in mind that advanced age and pre-existing CVD augment the probability of trastuzumab-induced cardiotoxicity and, thus, regular monitoring combined with prompt treatment are of utmost importance. Consequently, serial assessment of LVEF (via ECHO or other cardiac imaging tests) and measurement of specific cardiac biomarkers, as well as the use of medication, such as ACEI, angiotensin-receptor blockers, or BB, should be considered, together with a close follow-up (e.g., HF symptoms, arterial blood pressure, heart rate, LVEF, and renal function parameters). Such a proactive approach will hopefully prevent several unnecessary cardiovascular complications.
Future studies are needed to determine whether the selected cardiac biomarkers, novel imaging techniques, or their combination can be successfully employed in the older population for early detection; timely treatment; and regular, long-term follow-up of trastuzumab-induced cardiac adverse effects. There is the necessity to switch the thinking pattern regarding cardiotoxicity from ‘responding to a problem’ to ‘preventing and successfully solving problems’. Finally, a future challenge is the establishment of relevant practice guidelines focussed on preventive and therapeutic strategies against cardiotoxicity at the dynamic ‘intersection’ of cardiology and oncology, with particular attention to a growing population of elderly patients.








