| Adverse events should be reported. For UK healthcare professionals, reporting forms, and information can be found at https://yellowcard.mhra.gov.uk/.Adverse events should also be reported to Accord-UK LTD on 01271 385257 or email [email protected].
For non-UK/EU healthcare professionals, you can report side effects directly via the national reporting system listed in Appendix V of the EU SmPC. |
Meeting Summary
Prostate cancer remains one of the most commonly diagnosed cancers worldwide, with 1.4 million new cases and over 375,000 deaths reported in 2022. These statistics reflect significant global disparities in incidence, screening practices, and access to treatment. Over the past decade, the clinical landscape of prostate cancer has rapidly evolved, largely due to the introduction of combination treatments utilising androgen deprivation therapy (ADT), a long-standing pillar in management, as a foundation.This article summarises presentations delivered during a symposium held on 28th November at the Global Congress on Prostate Cancer (PROSCA) 2024 in Vienna, Austria. Four globally recognised experts discussed recent advancements in prostate cancer treatment and how these impacted their clinical practice in 2024, providing unique perspectives and insights from key members of the multidisciplinary team (MDT) comprising urology, radiation oncology, and medical oncology.
Cosimo De Nunzio, Professor of Urology at the Sapienza University of Rome, Italy, and the meeting Chair, opened the meeting with an overview of the clinical landscape of prostate cancer in 2024. He highlighted the significant advancements in treatment options over the past decade and how these developments influenced his clinical practice in 2024. Athanasios Papatsoris, Senior Professor of Urology at the University of Athens, Greece, presented key highlights from 2024 from a urologist’s perspective. He critically analysed pivotal studies shaping prostate cancer care and concluded with an informative case study that illustrated how standard practices are evolving and emphasised the importance of MDT collaboration in ensuring the best outcome for the patient. Thomas Zilli, Professor at the Oncology Institute of Southern Switzerland, explored advancements in the management of localised and recurrent prostate cancer. He contextualised the implications of data from major clinical trials with a focus on optimising radiation therapy. Amit Bahl, Consultant Clinical Oncologist at the University Hospitals Bristol, UK, closed the presentations by highlighting transformative developments in the treatment of metastatic hormone-sensitive prostate cancer (mHSPC) and metastatic castration-resistant prostate cancer (mCRPC). He focused on the progression from doublet therapy (ADT + androgen receptor-targeted agents [ARTA]) to triplet therapy (ADT + ARTA + docetaxel), supported by landmark trials such as PEACE-1 and ARASENS, as well as the UK National Institute for Health and Care Excellence (NICE) approval of the oral gonadotropin-releasing hormone (GnRH) antagonist, relugolix, and olaparib plus abiraterone.
Welcome, Introductions, and Objectives
At the Accord Healthcare-sponsored symposium, ‘Prostate cancer playback: Highlights of 2024’, held on 28 November 2024 at the PROSCA 2024 hybrid meeting in Vienna, Austria, speakers from Italy, Greece, Switzerland, and the UK came together to recap 2024 from the perspective of key members of the MDT in a series of interactive presentations and discussion sessions. The symposium aimed to help delegates contextualise and understand the implications of key studies, consider how best to optimise ADT therapy, integrate updates to clinical guidelines into practice, and prepare for anticipated data releases and changes in 2025 and beyond.
De Nunzio opened the meeting by discussing the incidence and mortality rates of prostate cancer worldwide, with 1.4 million new cases and over 375,000 deaths reported in 2022.1 He emphasised the need for collaboration to address the alarming discrepancy in mortality between the Western world and regions such as South America and Africa. De Nunzio stated that these disparities are largely driven by limited access to treatment and screening in these regions.
It was recognised that prostate cancer remains a key area of research, with a steady volume of new data and approved therapies and regimens constantly shifting the treatment paradigm to facilitate the effective management of prostate cancer, from initial diagnosis to progression to metastatic disease.2,3 Key developments include the use of ADT in combination with therapies such as novel hormonal agents (NHA),4,5 and the use of poly-ADP ribose polymerase (PARP) inhibitors in patients with mCRPC.3
De Nunzio highlighted a significant shift in his clinical practice with the adoption of prostate-specific membrane antigen-positron emission tomography/computed tomography (PSMA-PET/CT), guided by the latest European Association of Urology (EAU) recommendations.3 This imaging modality is now used by De Nunzio to evaluate his patients with high-risk localised or locally advanced prostate cancer, as well as for patients experiencing biochemical recurrence (BCR) after radical prostatectomy (RP).
2024 Highlights: As a Urologist
Papatsoris conducted an initial poll to gauge the audience’s preferred choice of locoregional imaging for high-risk patients. Approximately 60% of respondents selected multiparametric magnetic resonance imaging (mpMRI), a choice that Papatsoris also favoured. However, he noted that recent evidence may establish PSMA-PET/CT as the new standard of care.3
He went on to discuss the Next-Generation Trial, an investigator-initiated Phase II prospective validating paired cohort study, which investigated the use of PSMA-PET/CT versus mpMRI for locoregional staging of prostate cancer. Papatsoris highlighted that PSMA-PET/CT demonstrated improved diagnostic accuracy over MRI for locoregional staging of intermediate- and high-risk patients before RP.6 As such, Papatsoris speculates that PSMA-PET/CT could play multiple roles at different stages of the patient’s journey, including screening prior to diagnosis and treatment, as well as post-treatment and for follow-up in patients with non-metastatic castration-resistant prostate cancer (nmCRPC) and mCRPC.
‘How would you treat a high-risk patient following post-radical prostatectomy?’ This question was posed to the audience by Papatsoris, who referenced the APA-RP study.7 The study was an open-label, single-arm, Phase II study conducted in community urology practices in the USA. This study challenged the traditional approach of waiting for BCR to occur before intervening, showing that 12 cycles of apalutamide plus ADT (relugolix) is a viable treatment option for patients with high-risk localised prostate cancer who have undergone RP. This conclusion is based on the 100% confirmed BCR-free rate seen at 24 months and a testosterone recovery rate of 77% at 12 months post-treatment. The safety and tolerability of apalutamide plus ADT was also consistent with previous reports, with 22% experiencing Grade 3 of 4 events and no deaths being reported.7
Papatsoris next shared that real-world experience with relugolix combined with androgen signalling inhibitors (78% darolutamide, 14% abiraterone, 7% apalutamide, 1% enzalutamide) in advanced prostate cancer has recently been published.8 Overall, 100% of 152 patients achieved castrate testosterone levels, and 90% achieved levels of <20 ng/dL, while PSA levels declined by 90% in 75% of the cohort, and 91% achieved a decline of ≥50%. Overall, 7% of patients discontinued due to adverse events, three had major cardiovascular (CV) events, and there was no evidence of any drug–drug interactions.8
Papatsoris concluded his presentation with an illustrative case study of a patient with de novo mHSPC. An initial audience poll revealed a preference for treating de novo mHSPC with ADT plus ARTA, a choice that Papatsoris endorsed. The patient in question was a 71-year-old male with a history of CV disease, depression, gastritis, and a positive family history of prostate cancer. He presented to the accident and emergency department with urinary retention and renal impairment. Imaging (CT of the abdomen and pelvis) revealed enlarged lymph nodes and multiple bone metastases, while prostate-specific antigen (PSA) testing showed a significantly elevated level of 1,345 ng/mL.
Before receiving the biopsy results, Papatsoris prescribed relugolix, emphasising the importance of promptly initiating a GnRH antagonist for patients with pre-existing cardiovascular disease to rapidly achieve testosterone suppression.9,10 After assessing the patient’s performance status and clinical frailty, Papatsoris recommended ADT plus ARTA. However, the patient opted for darolutamide monotherapy, which ultimately proved successful. Despite this positive outcome, Papatsoris reminded the audience of the findings from the ARANOTE trial, which demonstrated that ADT plus ARTA is superior to ADT monotherapy in patients with mHSPC and is the preferred treatment regimen when possible.11
2024 Highlights: As a Radiation Oncologist
To provide context for two major clinical trials in localised prostate cancer and their clinical implications, Zilli began his presentation with one of two illustrative case studies. The first patient was a 70-year-old man with no family history of prostate cancer, a Karnofsky Performance Status (KPS) of 90, and a PSA level of 12 ng/mL. A biopsy confirmed prostate adenocarcinoma, and the patient presented with mild obstructive lower urinary tract symptoms. His medical history included a prior myocardial infarction, for which he had received three stents and was on clopidogrel. After consulting with the MDT and considering all factors, the patient was directed toward radiotherapy (RT) as the preferred treatment option. Following this introduction, Zilli engaged the audience by asking what type of fractionation they would recommend for this patient, with over 75% of respondents opting for moderate hypofractionation.
Zilli used the poll results to initiate a discussion on the use of extreme hypofractionation compared to moderate hypofractionation, supported by findings from the PACE trials.12,13 The 2-year results from the PACE-A trial and the 5-year results from the PACE-B trial provided key evidence for this debate. The PACE-B trial, which compared conventional RT with stereotactic body RT (SBRT) in patients with localised prostate cancer, demonstrated that SBRT was non-inferior to conventional fractionation in terms of biochemical and clinical failure.13 Additionally, both treatment approaches showed comparable safety profiles and quality-of-life outcomes. At the 2-year follow-up of the PACE-A trial, which compared surgery with SBRT in patients with localised prostate cancer, SBRT was found to improve urinary function and sexual dysfunction scores compared to surgery. The findings from the PACE-B and PACE-A trials have led to the inclusion of the SBRT ultra-hypofractionation regimen in the 2024 NCCN prostate cancer guidelines for patients with localised disease, marking a shift in standard practice.14
With the type of RT carefully considered, the next critical question is the choice of hormonal therapy. Over 50% of the audience favoured short-course ADT (4–6 months) with a GnRH antagonist. This approach is supported by data from multiple key trials, including the NRG/RTOG 9408 trial, which demonstrated a prostate cancer-specific mortality benefit with short-term ADT compared to no ADT.15 That a GnRH antagonist is preferred over an agonist is supported by evidence showing that degarelix improved lower urinary tract symptoms compared to goserelin.16 Additionally, data from a subset of patients in the Phase III HERO trial found that relugolix achieved testosterone levels below 50 ng/dL by Day 4 in a significantly higher proportion of patients versus leuprolide, with similar trends observed in other clinically relevant measures (Figure 1).17
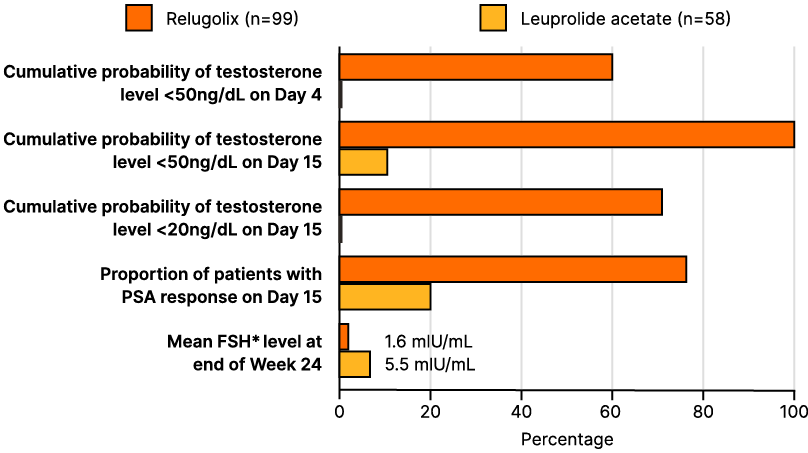
Figure 1: Overall survival by prostate-specific antigen response after doublet or triplet therapy in patients with metastatic hormone-sensitive prostate cancer.42
Phase III randomized controlled trials assessing treatment intensification with androgen pathway inhibitor, and/or docetaxel in patients with mHSPC were included.
*For CHAARTED, deep PSA response was reported at 7 months. LATITUDE trials reported PSA ≤0.1 ng/mL, which was assumed and defined as equivalent to PSA ≤0.2 ng/mL after the initiation of intensified therapy. TITAN trial reported PSA ≤0.2 ng/mL at 3, 6, and 12 months; however, the incidence of PSA ≤0.2 ng/mL at 6 months is reported here for consistency. To note, TITAN only provided OS data by deep PSA response at 3 months, hence this was used in the analysis. ARASENS trial reported PSA ≤0.2 ng/mL at 24, 36, and 52 weeks; however, the incidence of PSA ≤0.2 ng/mL at 24 weeks (6 months) is reported here and OS by deep PSA response at 6 months was used for the analysis.
FSH: follicle stimulating hormone; HR: hazard ratio; mHSPC: metastatic hormone-sensitive prostate cancer; OS: overall survival; PSA: prostate-specific antigen.
The preference for using a GnRH antagonist is based not only on its superior efficacy compared to GnRH agonists in these patients, but also on consideration of the patient’s prior cardiovascular history. A meta-analysis of 11 randomised trials demonstrated that GnRH antagonists are associated with a lower risk of major adverse cardiovascular events (MACE) and a non-significant reduction in overall mortality compared to GnRH agonists.18
Zilli concluded his first case study by opining that AI-based models can assist clinicians in carefully selecting patients who are most likely to benefit from treatment, thereby reducing the likelihood of overtreatment. This was evidenced by the NRG/RTOG 9408 study, wherein an AI-based model demonstrated that only 34% of patients benefitted from short-term ADT in reducing the risk of distant metastasis.19
The second case focused on BCR after RP, analysing two key data releases from the RADICALS-HD trial.20 He presented the case of a 65-year-old with a KPS of 100, no major comorbidities, and an initial PSA of 15 ng/mL. The patient underwent RP with extended lymph node dissection for prostate adenocarcinoma in January 2024, with histology showing a Gleason score of 5+4. The patient’s PSA score has a doubling time of 3.5 months, and PSMA-PET/CT came back negative.
As regards treatment, 30% of the audience opted for salvage RT alone, while another 30% recommended salvage RT plus 6 months of ADT therapy. Zilli observed that the mixed responses from the audience reflect the data from the RADICALS-HD study.20 When comparing RT alone versus RT plus short-course ADT (SCADT), no benefit in metastasis-free survival (MFS) was observed.21
Intriguingly, when comparing RT plus SCADT with RT plus long-course ADT, a small improvement in MFS was observed; however, this did not translate into an overall survival benefit.21 These results are reflected in discrepancies between guidelines, with the EAU not displaying a clear preference in ADT duration,3 while the European Society for Radiotherapy and Oncology (ESTRO) recommends short-term ADT for patients with a low-risk profile and long-term ADT in patients with a high risk of further progression.22
According to Zilli, when a patient wants to preserve sexual function, testosterone recovery should be factored into the treatment decision. Testosterone recovery has been shown to be influenced by ADT duration, baseline testosterone levels, age, and comorbidities.23 The impact of ADT duration on testosterone recovery was confirmed by the TRANSPORT meta-analysis, which showed that as the duration of ADT increased, the time to recovery to non-castrate testosterone levels also increased (Figure 2).24 Zilli concluded by highlighting that the use of relugolix has been shown to increase the cumulative incidence of testosterone recovery compared to leuprolide, as demonstrated in the Phase III HERO study.25 This establishes relugolix as the preferred treatment option in cases where preserving sexual function is a priority. However, the question of whether clinicians should extend the duration of SCADT by 1–2 additional months when used in combination with an antagonist requires further evidence.26
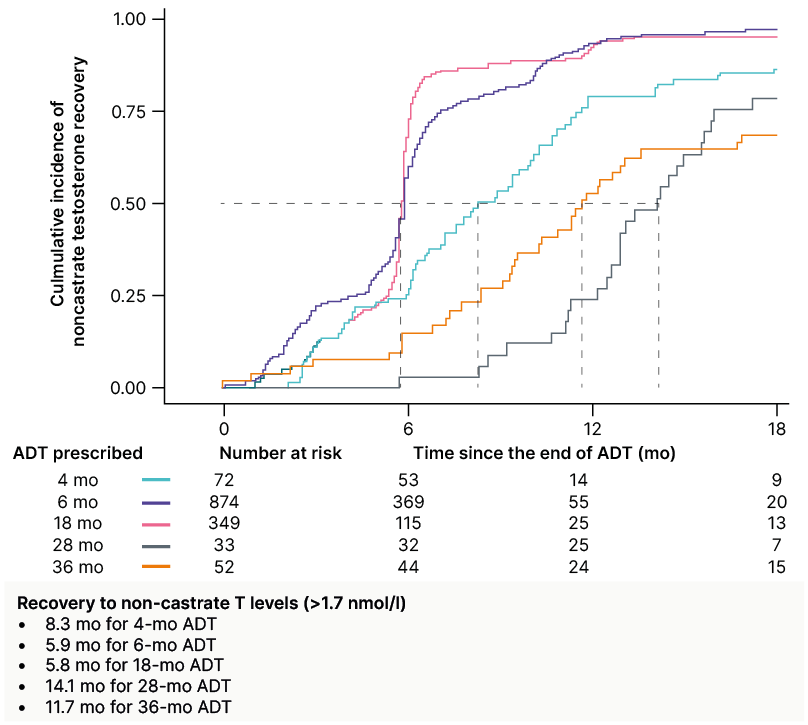
Figure 2: TRANSPORT meta-analysis study: the time to recovery to non-castrate testosterone levels (>1.7 nmol/L) increased with the duration of androgen deprivation therapy.24
Patient data from randomised controlled trials of radiotherapy with ADT was obtained and serial testosterone data from the MARCAP consortium was prospectively collected. The co-primary endpoints were time of TR to a non-castrate level (>1.7 nmol/L) and to a non-hypogonadal level (>8.0 nmol/L).
ADT: androgen deprivation therapy; mo: month; T: testosterone; TR: testosterone recovery.
2024 Highlights: As a Medical Oncologist
Bahl opened his presentation by stating that the last decade has seen transformative advancements in the management of mHSPC. From the STAMPEDED ARM C trial, which established docetaxel plus ADT as the standard of care, to the introduction of abiraterone through the LATITUDE and STAMPEDE ARM G trials,27-29 the field has evolved significantly. Recent doublet and triplet trials, such as PEACE-1 and ARASENS,30,31 have further integrated NHAs into the treatment paradigm. Importantly, at each turning point, we have seen significant improvements in outcomes, from a median overall survival rate of 2 years to 5–6 years; though, further improvements are required before mHSPC can be considered a chronic disease.32-35
Key trials, such as CHAARTED and ARANOTE,32,36 have firmly established doublet therapy of ADT plus ARTA as superior to ADT alone. Bahl pointed out that the median time to castration resistance in the control arm of all ‘doublet’ trials was approximately 11–12 months.32,33,36-38 Consequently, 50% of patients progress to mCRPC within a year, underscoring the inadequacy of ADT alone as a treatment strategy for these patients.
ENZAMET marked the first Phase III trial to add docetaxel to the combination of ADT plus ARTA, signalling the beginning of the triplet therapy era.39 Bahl discussed findings from the PEACE-1 and ARASENS trials, both of which demonstrated significant improvements in overall survival.40,41 In the UK, however, only the ARASENS triplet regimen (darolutamide + ADT + docetaxel) is approved for use and thus discussed in more detail by Bahl. Data from the ARASENS trial showed that, at 3.5 years follow-up, 62.7% of patients in the triplet group were alive compared to 50.4% in the ADT plus docetaxel group, representing an absolute difference of 12.3%.41 He noted that, despite this substantial improvement in overall survival, this result has not been met with the anticipated excitement and subsequent adoption in clinical practice. Bahl contrasted this with the management of breast cancer, where an absolute difference of 2–3% in survival is often deemed sufficient to justify chemotherapy.
When discussing which subgroups benefited most from triplet therapy in the ARASENS trial, Bahl emphasised the need for a shift in how the urology community classifies disease. He pointed out that the current classifications are “basic” and fail to include biomarker-based classifications, as seen in other oncology fields, such as breast and lung cancer. Despite this, time to castration resistance was prolonged in patients receiving triplet therapy (darolutamide + ADT + docetaxel) compared to ADT plus docetaxel alone in patients with high-volume/high-risk and low-volume/low-risk disease.42 However, an improvement in overall survival was observed only in patients with high-volume/high-risk disease, with no significant survival benefit seen in patients with low-volume/low-risk disease.42
PSA responses have been proposed as a potential marker for overall survival benefit following doublet or triplet therapy in mHSPC. Bahl highlighted findings from a Bayesian meta-analysis that showed a significant improvement in overall survival among patients with mHSPC who achieved a deep PSA response compared to those who did not (Figure 3).43 These findings showcase the utility of PSA response as a prognostic indicator and its potential utility in guiding treatment decisions.
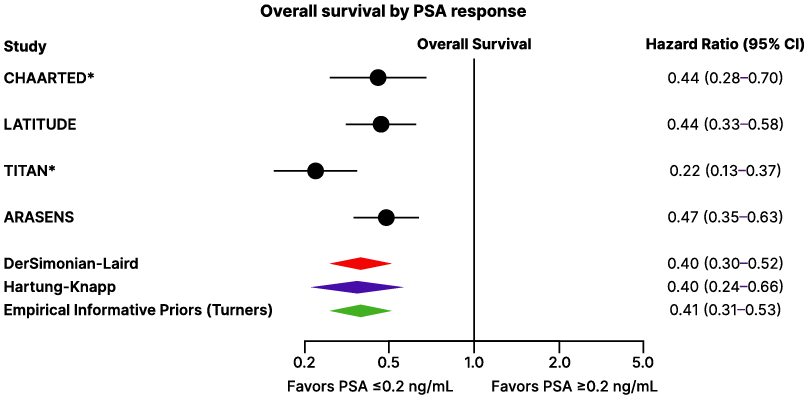
Figure 3: Overall survival by prostate-specific antigen response after doublet or triplet therapy in patients with metastatic hormone-sensitive prostate cancer.42
Phase III randomized controlled trials assessing treatment intensification with androgen pathway inhibitor, and/or docetaxel in patients with mHSPC were included.
*For CHAARTED, deep PSA response was reported at 7 months. LATITUDE trials reported PSA ≤0.1 ng/mL, which was assumed and defined equivalent to PSA ≤0.2 ng/mL after the initiation of intensified therapy. TITAN trial reported PSA ≤0.2 ng/mL at 3, 6, and 12 months; however, the incidence of PSA ≤0.2 ng/mL at 6 months is reported here for consistency. To note, TITAN only provided OS data by deep PSA response at 3 months, hence this was used in the analysis. ARASENS trial reported PSA ≤0.2 ng/mL at 24, 36, and 52 weeks; however, the incidence of PSA ≤0.2 ng/mL at 24 weeks (6 months) is reported here and OS by deep PSA response at 6 months was used for the analysis.
mHSPC: metastatic hormone-sensitive prostate cancer; OS: overall survival; PSA: prostate-specific antigen.
To conclude the discussion on the use of doublet and triplet therapy, Bahl conducted a series of polling questions. The first question asked the audience whether they would use systemic triplet therapy (ADT + docetaxel + ARTA) for different mHSPC cases (e.g., de novo high-volume/high-risk or low-volume/low-risk). Approximately 80% of respondents indicated they would use triplet therapy for a patient with de novo high-volume/high-risk mHSPC, while only 13% stated they would never use triplet therapy. The second question, which Bahl found particularly interesting, revealed that approximately 50% of respondents preferred apalutamide as the ARTA of choice in doublet therapy. The third and final question focused on the preferred ARTA for triplet therapy, with darolutamide emerging as the overwhelming favourite for this regimen.
Shifting focus to mCRPC, the PROpel trial evaluated olaparib plus abiraterone versus abiraterone alone as first-line therapy.44 The trial met its primary endpoint, with the combination therapy demonstrating a 39% reduction in the risk of progression or death compared to abiraterone alone in the all-comers population. Regarding overall survival, a more pronounced benefit was seen in the BRCA subgroup,44 fuelling an ongoing debate on whether to treat all comers, those with a homologous recombination repair defect mutation (HRRm), or only BRCA1/2 mutation-positive patients with a PARP inhibitor. Despite this controversy, and unlike the FDA, NICE has approved access for this patient cohort without the need for a confirmed BRCA1/2 mutation in an effort to reduce the need for testing.45,46
On the theme of NICE approvals, Bahl highlighted that relugolix for the treatment of hormone-sensitive prostate cancer was one of the fastest prostate cancer drugs to receive approval by NICE.47 Bahl believes this rapid approval reflects the strength of the data and the high unmet need within this patient population. In the UK, NICE has approved relugolix for patients with advanced hormone-sensitive prostate cancer, alongside radiotherapy for high-risk localised or locally advanced hormone-sensitive prostate cancer, and as neoadjuvant treatment before radiotherapy for high-risk localised or locally advanced hormone-sensitive prostate cancer.47 He noted that with the EU, relugolix is indicated only for advanced prostate cancer.
Bahl emphasised that, in addition to the favourable safety profile of relugolix, its efficacy should also be highlighted. Relugolix has shown a greater sustained castration rate (Figure 4A) and a higher mean testosterone level in a subgroup followed for testosterone recovery (Figure 4B) compared to leuprolide in the Phase III HERO trial.48 This established relugolix as an effective treatment option for patients with advanced prostate cancer. To conclude his presentation, Bahl sought to find out which statement regarding ADT most often applies to the audience’s practice, with over 50% of respondents preferring GnRH antagonists to GnRH agonists in their clinic.
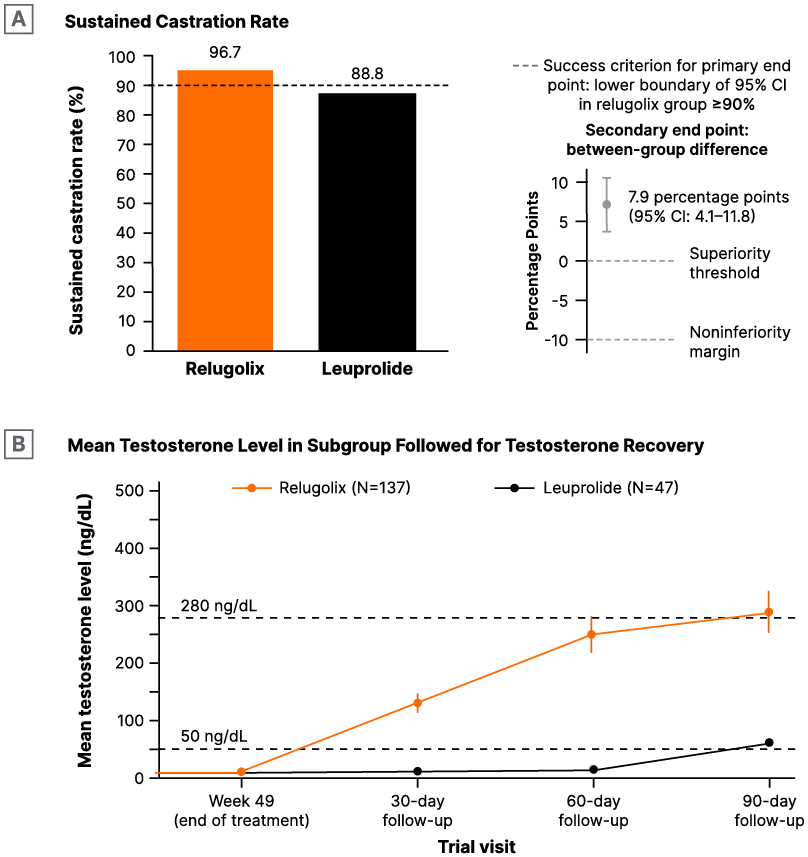
Figure 4: HERO study: Treatment with relugolix showed superiority over leuprolide in A) and B) higher mean testosterone levels following testosterone recovery.47
Multicentre post hoc analysis of patients in the Phase III trial of patients with advanced prostate cancer randomised in a 2:1 ratio, to receive relugolix (120 mg orally once daily) or leuprolide (injections every 3 months) for 48 weeks. The primary end point was sustained testosterone suppression to castrate levels (<50 ng/dL) through 48 weeks.
2025 Outlook: Panel Discussion
Following the final presentation, an open panel discussion moderated by De Nunzio provided attendees with the opportunity to have their questions answered by the esteemed panel. Bahl kicked off the discussion by addressing a question about the best candidate for doublet or triplet systemic therapy. He responded by stating that he assesses two key factors: 1) whether the patient is likely to die from metastatic prostate cancer, and 2) whether chemotherapy will ever be given to the patient. If the answer to both questions is yes, he opts for triplet therapy, as patients are more likely to tolerate the regimen at the start of their treatment journey, with sequential treatment options available if the patient relapses. Bahl reinforced the importance of using the best agents upfront.
In response to another question from an online attendee about whether ADT can be stopped in castration-resistant prostate cancer, Bahl clarified that this is possible by measuring testosterone levels, ensuring they remain in the castrate range. He emphasised that clinicians should always consider that the likelihood of testosterone recovery decreases the longer the duration of ADT. Zilli then shared his approach for patients at high risk of retention during radiotherapy. He prefers neoadjuvant ADT if the symptoms can be managed with it; if not, he refers the patient for surgery and waits 8–10 weeks before starting radiotherapy. Papatsoris closed the discussion by stating that RP still plays a role in patients with negative traditional imaging but a few spots on PSMA-PET/CT, followed by stereotactic treatment.
Concluding Remarks
De Nunzio concluded the meeting by speculating on the potential game-changers for 2025, beginning with a polling question about preferred screening programmes. The audience selected a combination of PSA, digital rectal exam, and MRI. De Nunzio then discussed the findings of a paper published by Hugosson J et al.,49 which showed that omitting biopsy in patients with negative MRI results eliminated more than half of diagnoses of clinically insignificant prostate cancer. This, he suggested, should be considered when discussing future screening programmes.
A discussion on game-changers in 2025 would be incomplete without mentioning AI. Results from a confirmatory study suggest that AI has the potential to serve as a supportive tool in the primary diagnostic setting.50 Another increasingly discussed topic is the use of novel nomograms in the PSMA era, with Gandaglia et al.51 proposing a new tool that offers better calibration and a higher net benefit compared to the other nomograms assessed.
Conclusion
The management and treatment of prostate cancer continue to evolve in response to unmet patient needs and to improve outcomes, with effective treatments now available from diagnosis through to advanced disease. The faculty provided valuable insights into managing localised, recurrent, and metastatic prostate cancer, highlighting how evidence from landmark clinical trials such as PEACE-1, ARASENS, and HERO has shaped current treatment strategies and influenced globally recognised clinical guidelines.
Summarising 2024 from a medical oncologist’s perspective, Bahl highlighted the rapid approval of relugolix by NICE, emphasising the significant unmet need within this patient population. The HERO trial demonstrated relugolix’s superior efficacy and favourable safety profile compared to the GnRH agonist leuprolide, offering a new, effective therapeutic option for these patients.
Looking ahead, innovations such as AI models show promise in aiding the diagnosis of prostate cancer and identifying patients who would benefit most from specific treatments. The use of AI models could help further personalise treatment strategies, reducing overtreatment and minimising unnecessary toxicity. With ongoing advancements in treatment options, screening techniques, and patients’ stratification methods, an MDT approach will remain essential in navigating the dynamic treatment landscape and continuing efforts to improve survival and quality of life for patients prostate cancer worldwide.
EUR-Onc-Org-01525 | March 2025







