Meeting Summary
The theme of the symposium was the London tube, which is famous for the expression ‘Mind the gap’; the symposium theme was tweaked to ‘Mind the debate’ with three debates that focussed on gaps in understanding of nocturia. Nocturia is a multifactorial medical condition with several components including nocturnal polyuria, reduced bladder capacity, and sleep disorders. Nocturia can be caused by comorbidities such as heart failure, diabetes mellitus, and sleep apnoea. The debate discussed that nocturia is a highly prevalent medical condition that increases with age and affects both men and women. Nocturia disturbs sleep and can seriously affect a patient’s quality of life. The condition also increases mortality by making patients more prone to falls and to fracture the head of the femur. Nocturia results in poor concentration at work and can lead to a loss of productivity. Assessments for nocturia were considered including frequency volume charts (FVC), urine albumin to creatinine ratios, peripheral oedema examinations, bladder diaries, and ultrasound testing. One treatment for nocturia has been desmopressin, but the risks of hyponatraemia have led to a reluctance to prescribe, especially in populations aged >65 years, who are at particular risk if treated with too high a dose. Recently NOCDURNA®, a gender-specific low-dose oral lyophylisate formulation of desmopressin, has been developed (50 µg/day in men and 25 µg/day in women). At these low doses desmopressin was shown to be effective and well-tolerated in two Phase III trials and to provide rapid and sustained improvements in nocturia and quality of life. The formulation is suitable for individuals >65 years old, but the advice is that they require sodium monitoring before initiating the treatment, in the first week of treatment (4-8 days) and again at one month after treatment initiation.
Introduction
The symposium involved three debates focussing on gaps in the understanding of nocturia: ‘Nocturia: why bother?’, ‘Is treating nocturia easy?’, and ‘Are all desmopressin formulations the same?’. Prof Philip Van Kerrebroeck, the chairman, outlined the evening’s format with experts providing medical evidence and sharing perceptions of nocturia.
Nocturia: Why Bother?
Doctor Jonathan Rees
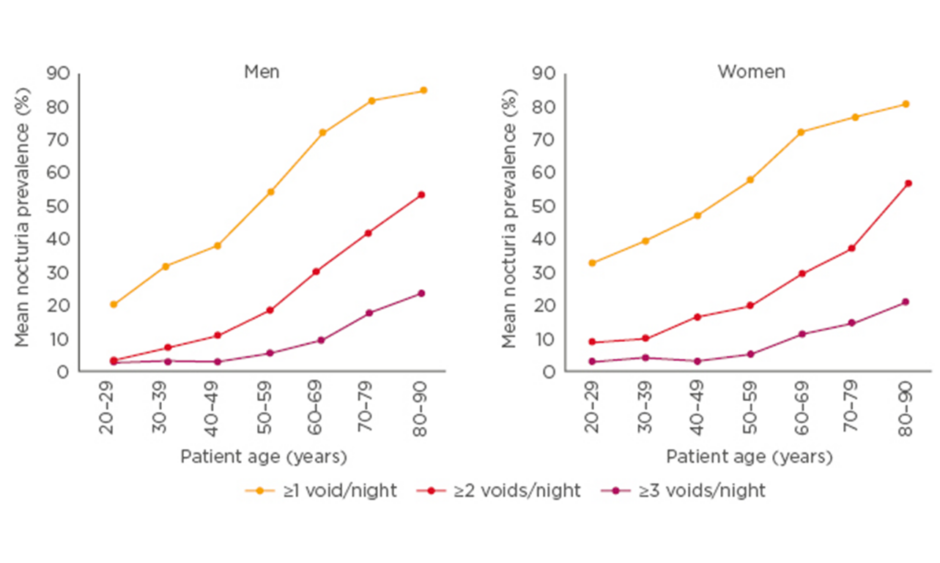
Nocturia can be considered a disease of ageing because it becomes more prevalent with age in both men and women. A systematic review and meta-analysis showed ‘having to wake at night ≥1 times to void’ affected around 55% of men aged 50–59 years, 70% aged 60–69 years, and >80% aged 80–90 years. Similarly, the analysis showed nocturia affected around 60% of women aged 50–59 years, 70% aged 60–69 years, and 80% aged 80–90 years.1 See Figure 1.
Figure 1: Nocturia is common across all age groups in both men and women.
Adapted from Cornu et al.1
While nocturia can be considered a normal aspect of ageing, clinicians should be careful not to ‘over normalise’ the condition. Short times to first void, said Dr Rees, have been associated with lower sleep quality. A study of 757 nocturia patients showed those in the lowest quartile of time to first void (<1.17 hours) were nearly three-times more likely to have Pittsburgh Sleep Quality Index (PSQI) scores indicating poor sleep (>5) than those in the highest quartile (>2.5 hours) (odds ratio [OR]: 2.96; 95% confidence interval [CI]: 1.75–5.01).2 Furthermore, two studies showed the number of patients reporting good sleep quality decreases in direct relation to increasing numbers of nocturnal voids.3,4 Generally, explained Dr Rees, restorative slow wave sleep occurs when people first go to sleep, making disrupted sleep in the first 4 hours the most detrimental.
Nocturia can impair quality of life, with effects including impaired daytime activity and work productivity (daytime fatigue, poor concentration, reduced performance at work, and increased sick leave) and reduced quality of life (daytime fatigue, lower perceived health, and depression).3,5 Furthermore, nocturia has been shown to increase cardiovascular disease (CVD), falls and fractures, depression, diabetes, and obesity.6
The burden of nocturia increases with frequency of voids. A study showed deterioration of health-related quality of life was associated with an increasing number of night-time voids (p<0.001) with significant differences observed between 0–1 voids and >2 voids (p<0.001).7 A meta-analysis of seven studies (N=28,220 patients) showed nocturia was associated with increased all-cause mortality (hazard ratio [HR]: 1.23 for all patients with nocturia; HR: 1.46 for patients with >3 voids).8 Fatigue due to nocturia results in poor concentration at work. An analysis by Prof Van Kerrebroeck across 15 EU countries estimated the total cost of nocturia per annum due to lost productivity was around €30 billion. 9 Increased mortality from nocturia was driven by the increased risk of fractured neck of the femur in elderly patients getting up at night. Dr Rees concluded the data were clear that nocturia represents a significant health problem and that it was important for clinicians to be ‘bothered’ by it.
In the accompanying debate, the panel agreed that important factors urologists should take into consideration included the number of episodes of nocturia experienced each night, the timing of the episodes, and the extent to which symptoms bothered patients. What really mattered for patients, it was felt, was whether the condition disturbed their sleep. Getting up twice in the night was generally felt to be the point at which disruption started to impair quality of life. Additionally, single episodes of nocturia had greater adverse effects if they occurred in the first 4 hours of sleep (where slow wave restorative sleep occurs) as opposed to the second 4 hours. Comorbidities that may be uncovered by nocturia include CVD, undiagnosed sleep apnoea, and diabetes. There was also evidence that patients with nocturia are at increased risk from falls, fractures, and mortality. In patients presenting with nocturia, Dr Rees stressed that urologists needed to take into consideration whether they were frail and at risk from falls.
While the audience reported transurethral resection of the prostate had been largely abandoned as the principal treatment for nocturia, the panel said that the procedure is still offered at some centres and general practitioners (GPs) still refer patients. Assessments offered to nocturia patients include FVC, basic blood tests, haemoglobin A1c (HbA1c) tests, renal function, urine albumin to creatinine ratios, blood pressure tests, and peripheral oedema examinations. While FVC provide valuable information, they can be cumbersome and can take 3 days to perform. It was felt that more GPs would be willing to offer such tests if they appreciated that even 1 day of monitoring provides valuable information. Sufficient time also needs to be spent explaining to patients how instructive the data can be. While recording drink volumes was largely unnecessary, Prof Van Kerrebroeck felt it was important to investigate why patients with very high urinary outputs were drinking excessively, as this might impact on therapy.
Is Treating Nocturia Easy?
Professor Marcus Drake
Nocturia can be considered a multifactorial medical condition with components including nocturnal polyuria, global polyuria, reduced bladder capacity, and sleep disorders (Figure 2).

Figure 2: Nocturia is a multifactorial medical condition.6,11
AVP: arginine vasopressin; BPH: benign prostatic hyperplasia; DO: detrusor overactivity.
Nocturia is primarily caused by nocturnal polyuria. One study demonstrated that 76% of subjects in a European cohort of 846 nocturia patients and 88% of subjects in a USA/Canadian cohort of 917 nocturia patients experienced nocturnal polyuria.10 Investigations to classify patients into the correct nocturia category include reviewing past medical histories; 3-day FVC; sleep questionnaires; fluid intake recordings; health-related quality of life questionnaires; and urine analysis, culture, and cytology.11 Fluid intakes need to be taken into particular consideration for patients with kidney stones or cystitis and those who exercise intensively, as both groups drink large amounts of water. FVC used to collect quantitative voiding data offer an essential evaluation tool for classifying nocturia. Global polyuria is defined as 24-hour urinary output that exceeds >40 mL/kg body weight while nocturnal polyuria is defined as >20% of daily urine output at night in young patients and >33% in elderly patients.12
The European Association of Urology (EAU) Non-neurogenic Male lower urinary tract (LUTS) Guideline Panel is working to develop a sensible approach that pulls ‘everything together’.13 Their guideline for evaluation includes history (including an assessment of sexual function), symptom score questionnaires, physical examinations, urinalysis, prostate-specific antigen (if prostate cancer diagnosis changes management), and measurement of post-void residual. In addition, said Prof Drake, ultrasound of the urinary tract may be needed to evaluate hydronephrosis and post-void residuals, and bladder diaries should be considered for patients with ‘bothersome’ nocturia. Patients with nocturia that is not ‘bothersome’ should still be assessed because this offers the chance to pick up other medical conditions at an early stage. He stressed that people with comorbidities, such as heart failure, should then be steered to appropriate specialists.
Management can then be tailored depending on the aetiology of nocturia:11,14
- Nocturnal polyuria: patients can be advised to reduce fluid intakes, be prescribed therapy for specific medical conditions, be prescribed desmopressin, and advised to change the time they take diuretics.
- Global polyuria: patients can be advised to reduce fluid intakes and be treated for diabetes mellitus/insipidus.
- Reduced bladder capacity patients can receive therapy for overactive bladder/benign prostatic hyperplasia and other urological conditions.
In nocturia treatment, little response has been seen for anticholinergic agents and α-blockers, Prof Drake cautioned. One study involving 997 patients with nocturia due to nocturnal polyuria found no significant difference in the number of nocturia episodes from baseline in those treated with the anticholinergic agent solifenacin 10 mg (n=400), solifenacin 5 mg (n=211), and placebo (n=386).15 Furthermore, in a Malaysian study involving 41 patients, 85% of patients with nocturnal polyuria were unresponsive to α1-blocker treatment compared to 10% with global polyuria and 5% with normal nocturnal outputs.16
In the accompanying debate, the panel considered whether patients with nocturia can be effectively treated with an α-blocker and/or an anticholinergic agent. Since nocturia can be caused by a combination of storage lower tract symptoms and bladder outflow obstruction, Dr Rees felt patients with mixed symptoms would respond to α-blockers while those with storage problems would respond to anticholinergic agents. Evidence from the COMBAT17 and STAR18 studies, he added, showed α-blockers used in combination with anticholinergic agents produced statistically significant improvements in nocturia. Concerns were expressed that initiating combination treatments together might make it difficult to identify which drug caused side effects. Instead, Dr Rees said, he preferred to start in a ‘step-wise fashion’ targeting the most prominent symptom first. Bladder diaries, where patients score each void, can provide valuable information to decide whether patients might respond to anticholinergic agents. Patients with 80 mL nocturia voids with symptom scores of 3 were felt to provide the perfect candidates for anticholinergic agents taken before bed.
The audience agreed diuretic treatments in male patients with significant nocturia should be considered a waste of time and money. While national UK guidelines advise loop diuretics for nocturnal polyuria,19-21 none of the panel recalled seeing any patients who had responded. An added complication was that, in addition to nocturia, patients receiving diuretics experienced evening diuresis. The current approach to nocturia assessment, involving multiple appointments, leads to delays of around 1 year before patients receive treatment. To prevent patients from becoming disillusioned, Prof Drake suggested that primary care was the most appropriate place to start α-blockers.
Are All Desmopressin Formulations the Same?
Professor Phillip Van Kerrebroeck
Recently a gender-specific low-dose melt formulation of desmopressin has been developed. Prof Phillip Van Kerrebroeck explained that the lower dose for female patients was because women are more sensitive to antidiuretic treatments than men and can be treated as effectively on the lower dose.22,23
Studies in the USA and Canada showed that desmopressin 25 µg in women significantly reduced the mean number of nocturnal voids compared to placebo (p=0.028);23 and that desmopressin 50 µg in men significantly reduced the number of nocturnal voids compared to placebo (p<0.001).24 See Figure 3.
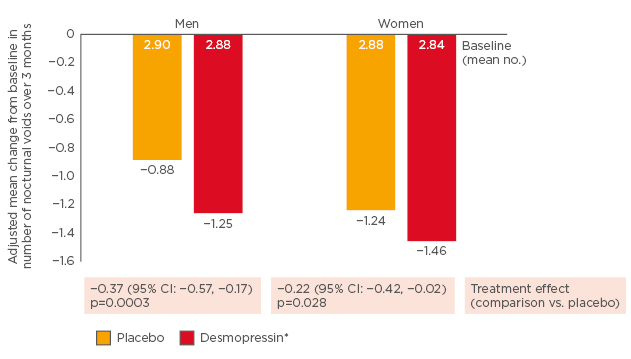
Figure 3: Significant reductions in number of nocturnal voids with low-dose desmopressin.23,24
*Men: 50 µg; women: 25 µg.
CI: confidence interval.
In women, desmopressin 25 µg increased the time to first nocturnal void by 49 minutes compared to placebo (p=0.003),23 while in men desmopressin 50 µg increased time to first nocturnal void by approximately 40 minutes compared to placebo (p=0.006).24
The first period of uninterrupted sleep is important, explained Prof Van Kerrebroeck, because it correlates with feeling well rested. This view was enforced by studies showing significant increases in health-related quality of life after 3 months treatment.23,24 Low-dose desmopressin was well-tolerated, with only two women23 and three men24 having adverse effects leading to discontinuation. Furthermore, only 2% of women and 2% of men experienced serum sodium levels <130 mmol/L.23,24
The authors of a recent meta-analysis of low-dose desmopressin studies recommended monitoring at Week 1 and additional monitoring at Month 1 for those at elevated risk (aged >65 or receiving concomitant medication associated with hyponatraemia).25 Furthermore, the product characteristics summary for NOCDURNA® points out that for elderly patients, serum sodium must be in the normal range before initiating treatment and checked at 4–8 days and Month 1.26 Low-dose desmopressin, concluded Prof Van Kerrebroeck, provides a good balance between efficacy and side effects (in particular hyponatraemia) and offers a welcome addition to the nocturia therapeutic armamentarium.
High-dose desmopressin, the panel acknowledged in the accompanying debate, could have significant health implications for patients with low sodium levels (<130 mmol/L). The reason UK guidelines recommend trials of loop diuretics,19-21 said Dr Rees, is that experts have not considered high-dose desmopressin to be “safe and viable”, with risks especially pronounced for older patients. The introduction of new formulations of lower-dose gender-specific desmopressin (50 µg for men and 25 µg for women) significantly reduces the risk of hyponatremia compared with higher doses.
Since risks of hyponatraemia are greatest in patients aged >65 years, the panel felt it should be considered obligatory to investigate serum sodium in this age group before starting desmopressin. Levels should then be tested again after 7 days. If there are no major changes in the patient’s medical condition there is no need for chronic monitoring, although tests should be repeated if the dose of desmopressin is changed. Patients and their relatives need to be warned that sodium should be measured if they become acutely unwell. When prescribing to elderly patients with significant comorbidities, Prof Van Kerrebroeck suggested it was helpful to involve geriatricians. Sublingual preparations, commented Prof Drake, can represent an advantage for nocturia patients since they reduce liquid consumption at bed time. Patients should also be provided with advice to maintain stable diets that do not involve reduced sodium intakes and not to increase fluid intakes. Even with the new formulations, it was felt, the advice should be to limit fluid intakes to 500 mL for an hour before bed.
Conclusion
The symposium considered how nocturia, a highly prevalent medical condition, disturbs sleep, adversely affects quality of life, and increases mortality by making patients more prone to falls. Additionally, comorbidities that may be uncovered by nocturia include CVD, sleep apnoea, and diabetes. For nocturia treatment, limited response has been seen for anticholinergic agents and α-blockers, and, while more effective, there have been concerns high dose desmopressin may be associated with hyponatraemia. Recently, new gender-specific formulations of low dose desmopressin (50 µg/ day in men and 25 µg in women) have been developed to overcome issues with hyponatraemia. The new formulations have been shown to be effective in two Phase III clinical trials.