Meeting Summary
Prof Per-Uno Malmström opened this symposium on non-muscle invasive bladder cancer (NMIBC) by describing the medical and economic burden caused by the increasing incidence of bladder cancer and the lack of new therapeutic options available to address the challenges of the management of NMIBC. Prof Marko Babjuk followed with a presentation that demonstrated that risk stratification using European Organisation for Research and Treatment of Cancer (EORTC) and Spanish Urological Club for Oncological Treatment (CUETO) risk scores remains a useful tool for determining the best individual treatment options for patients. The next presentation, given by Dr Carsten Ohlmann, described the use of mitomycin C (MMC) for low and intermediate-risk patients as per the European Association of Urology (EAU) guidelines. However, despite a favourable safety profile, single case reports of severe adverse events following treatment with MMC should not be dismissed. MMC should therefore be given with care, with an emphasis on performing high quality transurethral resection of the bladder (TURB). Prof Bernard Malavaud then presented details of newer diagnostic methods, such as photodynamic diagnosis (PDD) and narrow band imaging (NBI), which offer better optical tumour recognition for the surgeon than the old standard of white light cystoscopy. The uptake of PDD and NBI in the future will facilitate an increase in the quality of TURB. Finally, Prof Ashish Kamat explained that recurrence of bladder cancer after bacillus Calmette–Guérin (BCG) treatment (‘BCG failure’) needs to be more clearly defined and stratified. He stated that optimal recognition of timing with relation to BCG immunotherapy is critical to determine the next steps. For example, in the past, patients with late recurrence who may have benefitted from challenge with BCG may have been overlooked.
Introduction
Professor Per-Uno Malmström
Prof Malmström welcomed the audience to the MEDAC-sponsored satellite symposium on NMIBC. Based on the results of interactive polling, 51% of the audience worked in a university hospital, 24% in a regional/local hospital, and 25% in a private office/hospital. Sixty-two percent of participants knew their hospital’s recurrence rate after TURB: this was 26–50% for the majority (61%) of respondents. The audience voted on treatment options for two case studies at both the beginning and end of the symposium.
The Changing Epidemiology of Bladder Cancer
Professor Per-Uno Malmström
There are approximately 120,000 new cases of bladder cancer and 40,000 deaths per year in the European Union. Disease rates have been rising steadily in both males and females since the 1960s due to the increasing numbers of elderly people in the general population. Data from the Swedish National Registry shows an increase in non-invasive papillary carcinoma (Ta) tumours with a slight decrease in T2–T4 tumours, whereas in the USA there has been a dramatic increase in Ta tumours among the oldest age strata. Over a 5-year follow-up period, a 48% recurrence rate was seen in Sweden, mainly occurring during the first year, with a progression rate of 8%. Further data from the Swedish National Registry shows that bladder cancer survival rates remained largely unchanged between 1997 and 2011 in contrast to colorectal cancer survival rates, which increased over the same period. Bladder cancer represents 5% of total cancer healthcare costs, and productivity losses and informal care represent 23% and 18% of bladder cancer costs, respectively.1 In summary, the current challenges in the management of NMIBC are: the rising incidence, especially among the elderly, the unacceptably high recurrence and progression rates, the static survival rates, and a lack of new drugs.
Case Study 1
Q1) How would you manage a 60-year-old female with a history of Ta low-grade, now presenting with multiple recurrent tumours at follow-up white light cystoscopy? You think she would not accept coagulation in the office. What would you do?
- TURB in white light
- TURB in blue light
- TURB with NBI
Results of the voting at the beginning and at the end of the symposium are shown in Figure 1.
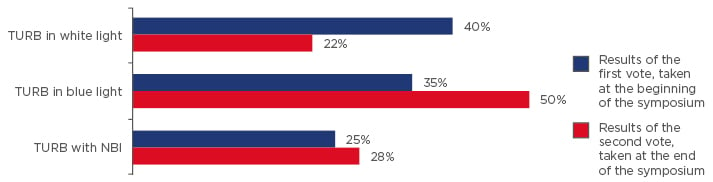
Figure 1: Results of questionnaire (question 1, case study 1); vote taken at the start and again at the end of the symposium.
The speakers posed the question “how would you manage a 60-year-old female with a history of Ta low- grade, now presenting with multiple recurrent tumours at follow-up white light cystoscopy? You think she would not accept coagulation in the office. What would you do?”
TURB: transurethral resection of the bladder; NBI: narrow band imaging.
Q2) Do you give single-shot postoperative chemotherapy after uncomplicated resection?
- Yes
- No
72% of the audience voted yes.
Case Study 2
Q3) How would you manage a 71-year-old male, T1 high-grade, diagnosed 3 years ago? He completed 2 years of BCG and had a good result. Now cystoscopy shows a red area and the biopsy shows carcinoma in situ (CIS). You recommend cystectomy but he has a cardiac condition and he is very negative to major operations. Which of the alternatives do you suggest?
- New BCG course
- MMC
- Device-assisted mitomycin administration
Results of the voting at the beginning and at the end of the symposium are shown in Figure 2.
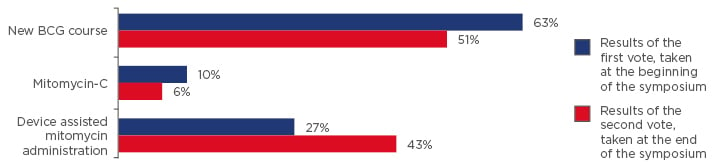
Figure 2: Results of questionnaire (question 3, case study 2); vote taken at the start and again at the end of the symposium.
The speakers posed the question “how would you manage a 71-year-old male, T1 high-grade, diagnosed 3 years ago? He completed 2 years of BCG and had a good result. Now cystoscopy shows a red area and the biopsy shows carcinoma in situ. You recommend cystectomy but he has a cardiac condition and he is very negative to major operations. Which of the alternatives do you suggest?”
BCG: bacillus Calmette–Guérin.
Do We Have Tools for Individualised Patient Treatment in Intermediate-Risk Non-Muscle Invasive Bladder Cancer?
Professor Marko Babjuk
When considering the treatment options for a 60-year-old female with multiple small papillary tumours on a control cystoscopy with a history of Ta low-grade tumour, there are three critical treatment steps that must be discussed: 1) endoscopic surgery, 2) early instillations of chemotherapy, and 3) further instillations. This session covered the first and third options. The EAU guidelines state that “In patients with a history of small, Ta low-grade (LG/G1) tumours, fulguration of small papillary recurrences on an outpatient basis can reduce the therapeutic burden and can be a treatment option.” However, as not all tumours are suitable candidates and as this is not routine practice in many countries, TURB using endoscopy under anaesthetic should be considered. The goal is complete removal of all lesions and a reliable diagnosis based on pathology. Newer imaging techniques such as PDD can increase the visibility and reduce the risk of missing small recurrences. Data published several years ago showed pronounced benefits in patients with multiple lesions and recurrent tumours using TURB with fluorescence cystoscopy compared with white light cystoscopy.2
A meta-analysis of Hexvix® stratified according to risk group showed a lower risk of recurrence in the intermediate-risk group, together with a less pronounced, but still significant, benefit of PDD than in the high or low-risk groups.3 NBI is another promising technique, albeit one with a paucity of published data at this time.
For patients with intermediate-risk tumours (with or without immediate instillation), guidelines state that: “1-year full-dose BCG treatment (induction plus 3-weekly instillations at 3, 6, and 12 months), or instillations of chemotherapy (the optimal schedule is not known) for a maximum of 1 year is recommended. The final choice should reflect the individual patient’s risk of recurrence and progression as well as the efficacy and side effects of each treatment modality”.4 However, the question remains as to whether BCG or chemotherapy is the better option. This question was addressed in the FinnBladder I study,5 a 20-year follow-up of 89 patients with NMIBC (highly recurrent disease, mostly Ta and G1 tumours). Of the patients treated with MMC, 80% experienced recurrence, compared with 59.1% of those treated with BCG. There was no significant difference in progression and survival. A meta-analysis of 2,820 patients (74% intermediate-risk) with a 4.4-year follow-up demonstrated that there was a 32% reduction in the risk of recurrence with BCG, although MMC was better than BCG without maintenance.6 Once again, there was no significant difference in progression and survival. It is important to remember that BCG has a greater side-effect burden and owing to shortages is unavailable in many countries. In view of this, strategies to inform individual decision-making are highly desirable. Some promising work on genetic markers has been completed,7 but this has yet to reach the clinic. The EORTC calculator, based on patients not taking BCG therapy, enables physicians to estimate the risk of tumour recurrence and progression on the basis of a number of clinical variables: number of tumours; tumour size; prior recurrence rate; T category; CIS; and grade.8 In a patient with multiple recurrent small papillary tumours, this translates to a 62–78% risk of recurrence and a 6% risk of progression at 5 years. If the tumours are >3 cm, the risk of progression increases to 17%, placing the patient in the high-risk category. This shows that in patients with intermediate-risk tumours (in contrast with high-risk), recurrence appears to be a greater problem than progression.
The CUETO scoring model, which assesses patients treated with BCG, calculates a risk of tumour recurrence that is approximately 10% lower for intermediate-risk patients compared with those not receiving BCG therapy.9 A more recent algorithm recommends a sub-stratification of intermediate-risk patients by individual risk factors to provide a more personalised approach.10 For instance, using this approach, an intermediate-risk patient with large and/or multiple tumours that recur frequently should be considered as a high-risk case.
In conclusion, individual decisions can be made at almost all stages of treatment with intermediate-risk tumours; the quality of surgery is crucial and individual selection of intravesical treatment can be made using risk calculators.
Single Immediate Instillation of Chemotherapy After Transurethral Resection of the Bladder: Still a Valid Recommendation?
Doctor Carsten Ohlmann
Single instillation therapy is often recommended after white light TURB since, in some cases, there may be a small residual tumour or small tumours in other areas of the bladder, and there may be floating tumour cells that could re-adhere to the bladder mucosa, giving rise to additional papillary tumours. The EAU guidelines of 2009 recommended immediate instillation in all NMIBC patients (low-risk, intermediate-risk [plus 1 year of BCG], and high-risk [plus 1 year of BCG]).11 These guidelines were based mainly on a meta-analysis published in 2004, which showed significant reductions in the risk of recurrence when using single immediate instillation (epirubicin, MMC, and pirarubicin).12 Single immediate instillation was shown to be beneficial in patients with single tumours (risk of recurrence was reduced by 11.3%) as well as those with multiple tumours (risk of recurrence was reduced by 16.3%), although additional adjuvant instillation therapy was also recommended owing to the high recurrence rate (65.2%).12 The current EAU guidelines now recommend immediate instillation therapy for low-risk and intermediate-risk patients only (plus 1 year of BCG or chemotherapy for intermediate-risk); no recommendation is made for high-risk patients.13 The lack of recommendation in high-risk patients is based on data that suggest that single instillation therapy is most effective in patients with single primary or small tumours, and patients with an EORTC risk score of >3 did not benefit from single immediate instillation of epirubicin.14 These findings were supported by a recent meta-analysis that also showed that single immediate instillation improves time to recurrence but not time-to-progression.15 Furthermore, patients with a low risk of recurrence (EORTC score 0) showed the greatest benefit from single immediate instillation therapy.15 There are some data showing a significant proportion of bladder perforation after TURB in patients who receive single immediate postoperative instillation therapy16,17 and in these patients there is evidence to suggest a higher rate of recurrence18 and a reduction in survival.15
In summary, the benefits of single immediate instillation therapy may have been overestimated in the past. Nevertheless, there are subgroups of patients who will benefit from single immediate instillation therapy, namely patients with single primary tumours and those with smaller tumours, but the issue of bladder perforation and related complications should be considered. In the future, improved TURB techniques and instillation therapies will reduce the use of single immediate instillation therapy.
Can New Imaging Techniques Improve Diagnosis and Follow-Up?
Professor Bernard Malavaud
At present, there are two main imaging systems for increasing the contrast between the cancer tissue and the surrounding normal tissues. One of those is PDD, which relies on highlighting deficiencies in haem synthesis, namely a cancer cell’s inability to metabolise hexaminolevulinate; this leads to an accumulation of protoporphyrin IX in cancer cells and causes the epithelium to become highly fluorescent. The other is NBI, which highlights microvasculature and the thickness of the mucosa using blue and green wavelengths of light. PDD enhances and facilitates the detection of papillary and flat high- grade lesions and a systematic review showed an increase in detection of 40% in CIS when using PDD.19 White light imaging (WLI) visualises all layers of the bladder wall and relies on the physician’s expertise in identifying variation in colour, contrast, and architecture, whereas PDD highlights cancer with a characteristic pink fluorescence. NBI relies on tumour angiogenesis;20 the green wavelength of light highlights the larger vessels and the blue wavelength highlights smaller vessels. CIS has a characteristic vascular pattern with a 10-fold increase in microvascular density easily detected by NBI. A further benefit of NBI is that the red light scattering that causes blurring in WLI is completely absent. However, red light is introduced in digital post-processing to enhance contrast in the image. A comparison of bladder imaging techniques is given in Table 1.
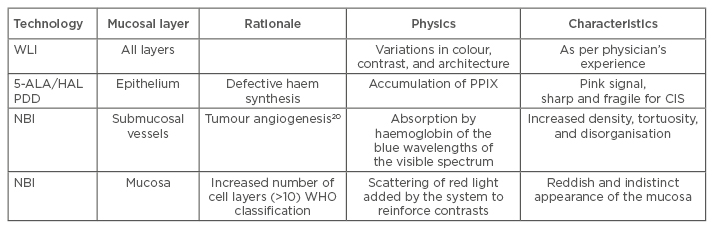
Table 1: Comparison of bladder imaging techniques.
5-ALA: 5-aminolevulinic acid; CIS: carcinoma in situ; HAL: hexaminolevulinate; NBI: narrow band imaging; PDD: photodynamic diagnosis; PPIX: protoporphyrin IX; WHO: World Health Organization; WLI: white light imaging.
A meta-analysis of 15 randomised controlled trials comparing WLI with PDD (n=11) and NBI (n=4) shows that there is a significant advantage in prevention of recurrence with both of the newer techniques (p=0.09 between subgroups).21 However, there was no impact on rates of progression with either new technique.21
Taking the example of case number 2 described previously, a 71-year-old male with a flat lesion at follow-up and a malignant cytology, it is necessary to exclude a tumour in the upper tract using computed tomography urography, a tumour in the prostatic urethra using a biopsy, and CIS in the bladder using random biopsies or PDD. The National Institute for Health Care and Excellence guidelines suggest offering white-light-guided TURB with one of either PDD, NBI, cytology, or a urinary biomarker test to such a case.22
In conclusion, new imaging techniques can improve diagnosis and follow-up. Both PDD and NBI are superior to WLI at the time of TURB and there is currently stronger evidence for PDD in the detection of CIS.
Bacillus Calmette–Guérin Failure: New Standardised Definition and Treatment Alternatives
Professor Ashish Kamat
Due to variations in endpoints and a lack of consensus on definitions of recurrence and/or progression of NMIBC, it is difficult to establish which treatments for bladder cancer are truly effective. A unified definition of BCG response and failure, with special attention to timing of the assessment, is therefore necessary to enable physicians and regulatory authorities to make informed decisions. For example, the landmark study of BCG by Lamm et al.23 showed a comparable complete response (CR) rate at 6 weeks in both the induction and maintenance therapy arms (58% and 55%, respectively). However, after 6 months of observation the CR rate in the induction-only arm increased to 69%, whereas in patients who had received an additional 3 weeks of maintenance treatment the CR rate increased to 84%. Taken together, these results suggest that of the patients who would have been considered ‘failures’ at 6 weeks, 64% could have been salvaged with an additional 3 weeks of BCG therapy. This is especially important in the context of clinical trials that report results after 6 weeks. These data also, incidentally, demonstrate that maintenance therapy is most beneficial with the following protocol: a 6-week induction followed by 3 weeks of maintenance at 3 months, 6 months, and every 6 months thereafter (known as the SWOG protocol). Other maintenance protocols, such as one instillation every 3 months or one instillation every month, fail to show a benefit24 and their use may represent one of the most common ‘errors’ in urology. In light of this, a definition of BCG failure must include a clear definition of adequate prior BCG therapy, namely BCG induction of 6 weeks plus at least one maintenance course of BCG of 3 weeks.25 Furthermore, BCG failure must be assessed at the 6-month time point after the diagnosis of a high-risk tumour, as continuing unsuccessful therapy after this point will expose the patient to unnecessary risks. A white paper seeking to unify definitions, end points, and clinical trial designs for NMIBC has recently been published.26 The term ‘BCG unresponsive’ has been adopted by the US Food and Drug Administration (FDA) and other agencies involved in clinical trial design. A BCG unresponsive patient is any patient with persistent high-grade disease at 6 months despite adequate BCG treatment, (including stage/grade progression at 3 months after instillation of BCG) or any patient with recurrence of high-grade disease within 6 months of last exposure to BCG (e.g. those on maintenance therapy). In order to provide consensus benchmarks for single arm studies, the following has been proposed by the International Bladder Cancer Group as evidence of ‘clinically meaningful benefit’: for BCG unresponsive CIS, an initial CR of 50% at 6 months; durable response rate of at least 30% at 12 months and 25% at 18 months; and for BCG unresponsive papillary disease, a recurrence-free rate of 30% at 12 months and 25% at 18 months.26
Radical cystectomy is the treatment recommendation for BCG unresponsive disease.4 However, not all patients are candidates for surgery and further options may be necessary. One option is repeated BCG; however, after two or more failed BCG courses the number of metastatic and invasive cancers overtakes the number of patients who are tumour free.27 A more recent study demonstrates that 71% of patients who have recurred with T1 disease while receiving repeated BCG therapy will progress to T2 disease, and almost half will be dead at 5 years.28 BCG plus interferon (INF)- may be a useful option in patients slower to fail BCG therapy; response rates when using this treatment were similar in BCG-naïve patients and patients who had failed BCG exposure >1 year earlier.29 A study of BCG versus IFN-2b plus epirubicin has shown that patients who have failed chemotherapy and then receive BCG can be salvaged more often than those who failed an initial course of immunotherapy.30 An open-label, Phase II trial of intravesical mycobacterium cell wall DNA complex achieved significant activity in high-risk NMIBC patients who were BCG unresponsive or in those whom BCG treatment failed, especially those with papillary-only tumours (61%, 1-year disease-free survival rate).31 Valrubicin is the only approved drug in the USA following BCG failure and shows a modest CR at 6 months of 18%.32 Gemcitabine after BCG showed promising early response rates ranging from 39–50% but the majority of patients recurred at 1 year, with a 1-year recurrence-free survival of 21%.33 Similar recurrence-free survival rates of 27.6% at 1 year and 21.0% at 2 years were seen in a SWOG protocol-based study of high-risk patients.34 Hyperthermic MMC post-BCG has shown demonstrable efficacy in a study of 111 patients with recurrent papillary NMIBC after BCG, with high disease-free survival estimates reported at 85% and 56% at 1 and 2 years, respectively; however, only 38% of patients were in the high-risk category and only 17% of patients had relapsed within 12 months of exposure to BCG.35 Combination therapy with gemcitabine and docetaxel has been shown to produce a treatment success rate of 32% at 2 years but results are dependent on the type of BCG failure.36 Emerging therapies include adenovirus IFN- gene therapy (instilladrin), which shows disease-free survival of 30% at 1 year,37 adenovirus vector CG0070 for granulocyte-macrophage colony-stimulating factor,38 FGFR3 inhibitors, mTOR pathway (everolimus plus gemcitabine), and EpCAM (vicinium). Despite these promising therapies, it is important to recognise that radical cystectomy is the treatment of choice in patients who have genuinely failed BCG or are BCG unresponsive, and that it should be instigated before patient disease progresses to T2.39
In conclusion, for patients with BCG unresponsive disease who refuse radical cystectomy (which still represents the ‘safest’ option), personalised therapy with directed use of instillation therapy as a salvage treatment can be offered.
Meeting Close
Professor Per-Uno Malmström
The results of the audience voting at the beginning and at the end of the symposium showed a shift in attitudes toward the use of TURB in blue light and device assisted MMC administration (Figure 1 and Figure 2).
The treatment of NMIBC is an important topic, one that is still in need of optimisation. Treatment should be tailored to the individual patient, and it does not necessarily follow that a patient with a recurrence after BCG treatment needs a cystectomy; if the recurrence is detected 2 years after BCG treatment, a rechallenge might prove effective. While single immediate instillation, chemotherapy, and immunotherapy are good options for controlling the recurrence and progression of these patients, the most important step in the management of NMIBC is an optimal TURB.








