Meeting Summary
Androgen deprivation therapy (ADT) has been used for many years for treating advanced prostate cancer (PCa) and remains the backbone of treatment. Luteinising hormone-releasing hormone (LHRH) receptor agonists are the most widely used ADT drugs. However, newer options, including gonadotropin releasing hormone (GnRH) receptor antagonists such as degarelix and relugolix, may be clinically more beneficial for some patients. GnRH antagonists reduce serum testosterone levels more rapidly than LHRH agonists, without an initial testosterone surge or subsequent microsurges.
This article summarises a symposium delivered on 11th March 2023 at the 38th European Association of Urology (EAU) Annual Congress in Milan, Italy, where speakers from three different disciplines described challenges and future perspectives for ADT in current clinical practice. Kurt Miller, Urologist, Charité University Hospital, Berlin, Germany, described the evolution of ADT in the treatment of PCa, from early reports of the benefits of surgical castration to the recent development of oral treatment for chemical castration. Miller explained the acceleration in progress in ADT research over recent years, with the development of novel drugs, drug sequences, and combinations, which have transformed outcomes in PCa. Alberto Bossi, Radiation Oncologist, Amethyst Group, Institut Gustav Roussy (IGR), Paris, France, next described current challenges with ADT management, including outstanding questions about the personalisation of ADT. Finally, Patrick Davey, Consultant Cardiologist, Northampton General Hospital, UK, spoke about ways to maintain a healthy heart on hormone treatment, and noted that cardiovascular safety is a major challenge in the use of ADT.
The meeting was chaired by Heather Payne, Consultant Clinical Oncologist, University College Hospital, London, UK, who introduced the speakers and co-ordinated a question-and-answer session at the end of the symposium.
Introduction
ADT, via medical or surgical castration, has been the mainstay of treatment for PCa for decades.1,2 ADT is currently most often achieved through the use of LHRH agonists such as leuprolide, goserelin, and triptorelin, which are administered subcutaneously or intramuscularly.1 These agents stimulate the LHRH receptor on Leydig cells in the testes, causing a temporary surge in testosterone levels before negative feedback, which results in receptor downregulation and leads to the suppression of testosterone release, reaching levels below castration (<50 ng/dL) within 4–6 weeks.1
More recently, GnRH antagonists such as degarelix and relugolix have been developed. These agents suppress the secretion of testosterone from the testes by binding competitively to the GnRH receptor in the pituitary gland, inhibiting release of the luteinising hormone and follicle-stimulating hormone, and thereby reducing downstream gonadotropin signalling.1 As GnRH antagonists do not cause an initial surge of serum testosterone, and can achieve castration levels within 2–3 days, they are proving to be a good therapeutic option for some patients with PCa.1 Relugolix also has the advantage that it may be administered orally, providing greater flexibility for patients.1
In the Accord Healthcare-sponsored symposium, ‘ADT in current clinical practice: Challenges and future perspective’, delivered on 11th March 2023 at the 38th EAU Annual Congress in Milan, Italy, speakers from three different specialties guided delegates through the development of ADT in PCa, from the earliest reports of the benefits of surgical castration in the 1960s to the generation of multiple new classes of ADT in recent years. Current challenges associated with ADT were also discussed, including questions around the combination of radiotherapy (RT) and ADT, and whether co-administration with a high dose of RT directly to the prostate may allow the duration of ADT to be reduced. Finally, the importance of cardiovascular (CV) safety in the use of ADT was considered, including an overview of the different levels of risk for different drugs, as well as insights into how to protect patients against the risk of CV events.
The Evolution of Androgen Deprivation Therapy in the Treatment Algorithm of Prostate Cancer
Kurt Miller
Miller opened the symposium by providing a short walk through the history of ADT, with its many twists and turns. They noted that many opportunities for learning had been encountered along the way, as is the case in most branches of medicine, but that progress had accelerated considerably in recent years.
The history of ADT for the treatment of PCa dates back to 1941, when Charles B. Huggins published their first paper on the effects of surgical castration (orchiectomy) on advanced carcinoma of the prostate gland.3 Huggins discovered that androgens, specifically testosterone, play a significant role in the growth and progression of PCa, and that suppressing serum testosterone levels increased life expectancy and reduced hours spent in pain.4 Huggins was eventually awarded the Nobel Prize for Medicine in 1966 for this pioneering work in treatment of advanced and metastatic PCa.1
Despite the successes of ADT, Miller noted that this treatment approach is not without side effects, including sexual dysfunction, hot flushes, osteoporosis/clinical fractures, metabolic syndrome, CV events, fatigue, depression, and sleeping disturbance.5 Miller noted that although these are not devastating consequences, both patients and physicians should be aware of the risk of these effects and how to manage them.
Following the initial work by Huggins, many studies evaluating different approaches to testosterone suppression for treating PCa were subsequently undertaken.1 Among these, the Veterans Administration Cooperative Urological Research Group (VACURG) studies conducted between 1960–1971 investigated the use of testosterone suppression using oestrogen in the form of diethylstilbesterol (DES).6 These studies showed that while a dose of 5 mg DES actually led to poorer survival outcomes compared with placebo, a 1 mg dose of DES increased survival rates compared with both higher DES doses and placebo.6 Miller commented that although they were still using oestrogens for castration-resistant PCa approximately 20 years ago, when there were no other alternative treatments, this treatment approach was largely discarded in the 1970s.
In the 1970s, another Nobel Prize was earned in this field when the father of LHRH agonists, Andrew Schally, discovered that serum testosterone levels were suppressed by constant exposure to LHRH, which downregulated the LHRH receptors, leading to desensitisation of pituitary cells.1,7 This discovery led to the development of LHRH agonists, which became a mainstream of treatment for PCa in the 1990s.1 Miller noted how the development of LHRH agonists led to the demise of orchiectomy for PCa, which had been the standard treatment in the 1970s and 1980s.
Miller next described how anti-androgens were introduced as new potential approach in the management of PCa. Instead of lowering testosterone levels, anti-androgen agents compete with androgens at the receptor level.8 Miller observed, however, that this approach never became mainstream as monotherapy for PCa, except in specific circumstances. Complete androgen blockade, using a combination of anti-androgens and LHRH agonists to block the androgens originating from both the testes and the adrenal gland, was subsequently proposed as a new approach to treatment for prostate cancer.9 The first reports indicated that complete androgen blockade may be able to cure at least 90% of cases of PCa, although subsequent studies revealed that the addition of an anti-androgen therapy improved absolute survival by approximately 2–3% only.10 Miller noted that the pursuit of effective complete androgen blockade represents another detour in the development of ADT for PCa.
The use of intermittent androgen blockade began to emerge as the next potential route in the management of PCa, on the basis that it may delay the onset of hormone resistance.11 However, no survival benefit was demonstrated for intermittent androgen blockade compared with continuous therapy, although some studies showed the results to be non-inferior and associated with some improvement in quality of life.11
The development of LHRH agonists and GnRH antagonists marked the beginning of a new era in the management of PCa.12 While LHRH agonists cause an initial testosterone surge that may result in a clinical flare of symptoms,13 GnRH antagonists have been shown to offer rapid reduction of serum testosterone without the initial testosterone surge and symptoms flare.1 The Phase III HERO study revealed rapid testosterone suppression to castration levels with the first oral GnRH antagonist, relugolix, which were then maintained throughout the treatment period.13 In contrast, the LHRH agonist leuprolide caused an initial surge in testosterone levels, before decreasing to and remaining at castration levels.13 The overall incidence of adverse events was consistent across treatment groups of the two agents,13 although there was a 54% reduction in risk of major adverse cardiovascular events (MACE) with relugolix versus leuprolide, as discussed later.14
Miller concluded their presentation by noting that little notable improvement in ADT efficacy was achieved for approximately 70 years following the early discoveries in 1941. However, progress rapidly accelerated after 2012, with multiple trials showing Level 1 evidence for improved overall survival (OS) in metastatic hormone sensitive PCa (Table 1). Miller noted that with new combination therapies, new drugs, and advances in cancer research, the future looks promising for patients with PCa undergoing ADT.
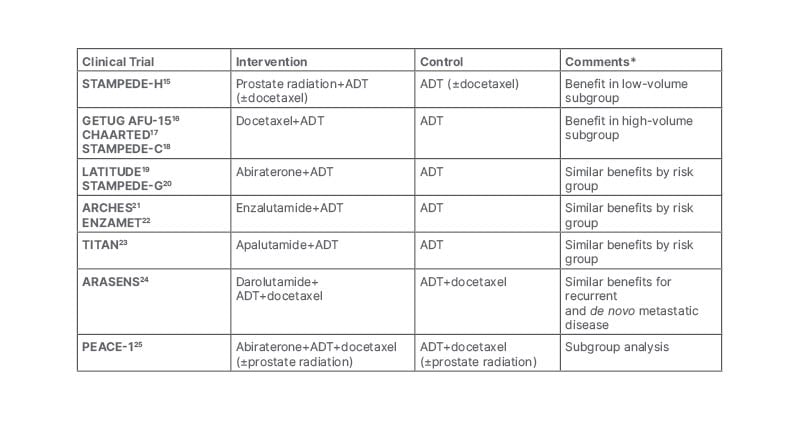
Table 1: Level 1 evidence for improved overall survival in metastatic hormone-sensitive prostate cancer.
*Comments reflect the views of the speaker.
ADT: androgen deprivation therapy.
Current Challenges with Androgen Deprivation Therapy Management
Alberto Bossi
Bossi gave the second presentation in the symposium, describing current challenges associated with the use of ADT, particularly in combination with RT. They noted that a high dose of RT is needed in the prostate to control the disease, irrespective of the method used for delivery, and the combination of RT and ADT gives better survival outcomes than either RT alone or ADT alone.26-31 However, Bossi noted that many of the studies are now several years old, and should ideally be repeated in the light of more recent developments in treating PCa. The findings of these studies are reflected in the current EAU Guidelines, which recommend long-term (2–3 years) ADT in high-risk patients with locally advanced disease and short-term (6 months) ADT in patients with unfavourable intermediate disease, while ADT is not recommended to treat patients with favourable intermediate disease.8
Despite all the evidence accumulated to date demonstrating the benefit of ADT and RT combination therapy in improving survival in PCa, Bossi noted that several unanswered questions remain. Among the issues that require further investigation is the current uncertainty around how long ADT should be administered with RT, and when treatment should be initiated. A meta-analysis of 12 randomised controlled trials (RCT), including more than 10,000 patients with intermediate- or high-risk disease, with median follow-up 11 years, confirmed that long-term ADT is better than short-term ADT in terms of metastasis-free survival or OS,32 as expected. However, they found no impact on metastasis-free survival or OS when adding neoadjuvant ADT to adjuvant ADT, meaning that there is no advantage in initiating ADT prior to RT. Bossi noted that this is contrary to common clinical practice, when patients may be given ADT while they are on the waiting list for RT therapy, or because physicians believe instinctively that this may offer some survival advantage.
Bossi commented that a second unanswered question in this area concerns whether delivering a high dose of RT directly to the prostate may allow the dose of ADT to be reduced, potentially improving the quality of life of the patient. A second meta-analysis, this time including 13 RCTs with more than 11,000 patients with intermediate- or high-risk disease and a median follow-up of 9 years, investigated the effect of low-dose RT (<74 Gy) versus high-dose RT (≥74 Gy), and short-term ADT (3–6 months) versus long-term ADT (18–36 months) on OS.33 This analysis revealed that long-term ADT rather than high-dose RT was the most important factor in improving both metastasis-free survival and OS, although there appeared to be some advantage to giving high-dose RT in terms of biochemical recurrence-free survival (Figure 1).
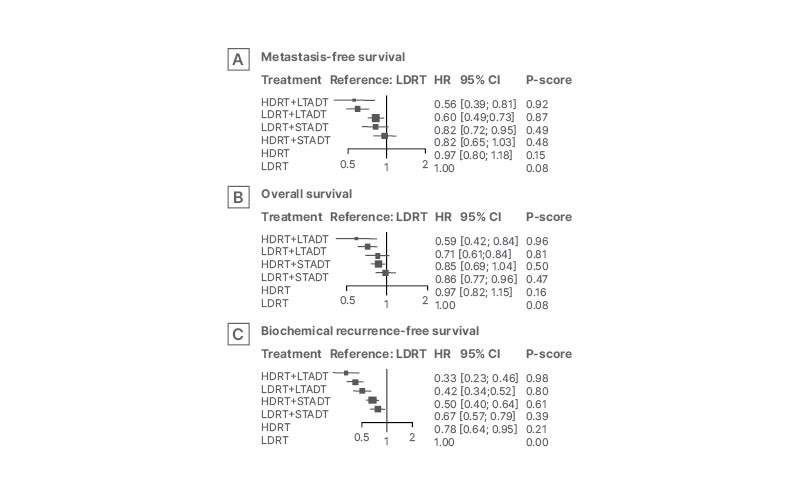
Figure 1: Forest plot derived from frequentist network meta-analysis of impact of treatment strategy on survival outcomes.33
Adapted from Kishan et al.33
CI: confidence interval; HDRT: high dose radiotherapy; HR: hazard ratio; LDRT: low dose radiotherapy; LTADT: long-term androgen deprivation therapy; STADT: short-term androgen deprivation therapy.
Bossi observed that one further challenge regarding the use of RT plus ADT in PCa was the relative toxicity of ADT. Although use of ADT is not recommended in low-risk PCa due to the lack of benefit and potential for harmful side effects, the National Cancer Database (NCDB) shows that a declining proportion of patients are still receiving ADT for low-risk PCa.34 Bossi noted that the best way to reduce ADT toxicity was to avoid ADT when it is unnecessary, for example as concurrent treatment for low-risk disease (including in preparation for local delivery of RT using brachytherapy). In addition, ADT should not be used as the sole primary therapy (without RT) for localised/high-risk PCa. Bossi described a potential strategy for reducing the overall requirement for ADT using brachytherapy (implantation of a sealed radiation source at the site where radiation is to be delivered) to boost the effect of externally-delivered RT, allowing a reduced duration of ADT.35 They also suggested that the use of ADT may be tailored through genetic profiling to create clinical-genomic risk groups that could be incorporated into treatment guidelines for localised PCa.36
Bossi concluded that ADT plays an essential role in the treatment of patients with high-risk PCa, and that combining it with RT has shown to have significant benefits in terms of OS. However, they noted that optimising the use of ADT to reduce toxicity and avoid unnecessary use remains an ongoing challenge for clinicians.
A Healthy Heart on Hormone Treatment (Androgen Deprivation Therapy)
Patrick Davey
The final talk given by Davey, a consultant cardiologist, focused on the increased risk of CV events with ADT and the impact of this risk on survival. They also considered differences in the risk of CV events between LHRH agonists compared with GnRH antagonists, and looked at ways to prevent CV adverse events, with guidance and practical tips for urologists.
Davey presented results of a Swedish nationwide population-based study showing that there was considerable CV disease (CVD) across the whole spectrum of disease among males with localised PCa, irrespective of PCa risk category or presence of comorbidities.37 Indeed, for low-risk PCa, PCa itself was actually only the third most common cause of death (18%), after CVD (31%) and other cancers (30%).
Among patients with PCa and pre-existing CVD, those with coronary artery disease have a small but definite increase in the risk of death (adjusted hazard ratio [HR] versus no coronary artery disease or stroke: 1.05; 95% confidence interval [CI]: 1.00–1.10), while patients who have had a stroke have a much greater increase in the risk of death (adjusted HR: 1.20; 95% CI: 1.12–1.30), possibly because of the greater functional impact associated with stroke compared with coronary artery disease.38 Even in patients with metastatic PCa, CVD still plays a significant role in determining mortality rates, as shown by data from the Surveillance, Epidemiology, and End Results (SEER) programme.39 Among 26,168 males with metastatic PCa, 16,732 died during the observation period (2000–2016), including 13,011 deaths from PCa and 1,147 deaths from CVD (standardised mortality ratio: 1.34). However, the SEER database does not include data on ADT exposure.39
In a real-world study of patients with metastatic PCa receiving conventional ADT, in which 32% of patients had pre-existing CVD, mortality rates were 50% higher in those with established CVD (14.8% versus 9.8% overall), while CV death also increased with age and increasing Charlson Comorbidity Index (CCI).40 This study showed that between the lowest and highest comorbidity cohorts, there was no change in PCa-specific mortality, a four-fold increase in CV mortality, and a two-fold increase in other-cause mortality, demonstrating the importance of preventing CV events in these patients.
Although early PCa drug treatments, including oestrogen, showed promise in improving PCa, any survival benefit was undermined by an increased CV risk.6 Concerns over CV risk in new generations of therapy became increasingly prominent from around 2005 onwards. Interestingly, observational studies tend to show an increased risk of MACE with ADT, mostly LHRH agonists, although the same risk is not always apparent in RCTs.41 For example, a population-based study from Sweden showed an increased relative risk of non-fatal and fatal CVD among all males with PCa, especially those treated with ADT.42 The reasons for the apparent difference between population-based studies and RCTs in this regard are not entirely clear, and may be due to inclusion criteria for RCTs (patients with high CV risk are more likely to be excluded) or reporting bias.
There is evidence to suggest that GnRH antagonists may be associated with a lower CV risk than LHRH agonists. For example, a 2014 pooled analysis of six Phase III trials of degarelix versus an LHRH agonist showed lower CV risk with the GnRH antagonist versus LHRH agonists in males with pre-existing CVD (HR: 0.44; 95% CI: 0.26–0.74; p=0.002).43 A similar finding was reported in a small prospective Israeli study, which showed that patients treated with an LHRH agonist experienced significantly more major CV and cerebrovascular events than those treated with a GnRH antagonist.44 However, in the PRONOUNCE study, the first international RCT to prospectively compare the CV safety of a GnRH antagonist and a LHRH agonist in patients with PCa, no difference in MACE was observed at 1 year between patients assigned to degarelix versus leuprolide, although the study was stopped early due to poor recruitment.45 Davey made the point that all patients in the study had seen a cardiologist, which may have improved CV safety in both groups and obscured any difference between groups. More recently, the HERO trial, described previously, showed an approximate halving of risk of MACE in the relugolix group compared with the leuprolide group.14
Overall, Davey concluded that CVD is common in PCa and worsens prognosis, and that LHRH agonists probably increase MACE, with the greatest increase in risk seen among those with pre-existing CVD. They also noted that GnRH antagonists are probably associated with lower CV risk than LHRH agonists, and concluded their presentation by considering the actions that can be taken to prevent CV events. Davey noted that management of CV risk should be based on lifestyle changes, exercise, strategies to reduce blood pressure and cholesterol, aggressive intervention for known CVD, and referral to a cardiologist for CV symptoms. They also presented the ABCDE of CV risk management: awareness and aspirin; blood pressure; cholesterol, cigarettes, and cardiologist; diet and diabetes; and exercise. Davey finally presented the European Society of Cardiology (ESC) guidelines, which offer specific recommendations for baseline risk assessment and monitoring during ADT for PCa (Table 2).46
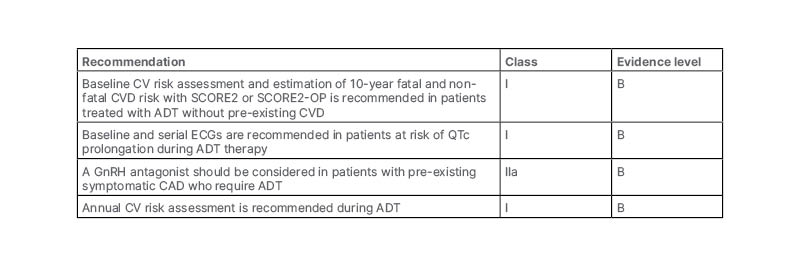
Table 2: Recommendations for baseline risk assessment and monitoring during androgen deprivation therapy for prostate cancer.46
Adapted from ESC 2022.46
ADT: androgen deprivation therapy; CAD: coronary artery disease; CV: cardiovascular; CVD: cardiovascular disease; GnRH: gonadotropin releasing hormone; QTc: corrected QT interval.
Conclusion
The presentations given in this symposium summarised the progress that has been made in ADT for PCa since the 1940s. It was noted that after the initial Nobel Prize-winning discovery of the benefits of surgical castration in prolonging life in patients with PCa, the rate of progress in terms of new treatments and innovations remained slow until 2012, when there was a sudden rush of new treatments developed and approved, which have offered significant benefits to patients since. However, despite the recent advances, certain questions and issues remain, which will need to be resolved in the future. For example, the relative safety and effectiveness of LHRH agonists and GnRH antagonists will need to be explored further, as well as the role of ADT in combination with RT. Improvements in genetic profiling may help determine which patients are likely to receive the maximum benefit from ADT, meaning that those unlikely to derive a clear benefit may be spared from the potentially harmful side effects. Finally, more work remains to be done to assess CV safety in ADT, in creating strategies to mitigate the risk, and developing new treatment protocols with CV risk at the centre.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard.
Adverse events should also be reported to Accord-UK LTD on 01271 385257 or email [email protected].
UK-04989 – April 2023







