Meeting Summary
Most prostate cancer cases present as localised disease at initial diagnosis but can progress in about a fifth of patients to castration-resistant prostate cancer (CRPC) within 5 years. A major concern for patients and physicians is the development of metastases, affecting quality of life (QoL), and reducing overall survival (OS). Treatment guidelines for the different stages of prostate cancer continue to be modified with the publication of clinical trial results. Currently, androgen receptor inhibitors (ARi) are used in the management of non-metastatic CRPC. Among these, explained Martin Bögemann, Department of Urology, University of Münster, Germany, darolutamide’s unique structure means it causes minimal side effects, likely due to reduced blood-brain barrier penetration, while also reducing the potential for drug-drug interactions, which is especially important for patients treated for comorbidities. Treatment of metastatic hormone-sensitive prostate cancer (mHSPC) depends on a variety of factors, including when metastases developed in the course of disease and their volume. Bertrand Tombal, Institute of Experimental and Clinical Research, Université Catholique de Louvain, Belgium, described the evolution of mHSPC therapy from androgen deprivation therapy (ADT) and surgical castration as the only available options, to the emergence of the chemotherapeutic docetaxel and androgen receptor pathway inhibition (ARPI). He explained the results of Phase III clinical trials of various combination approaches, with a combination treatment of docetaxel, ADT, and darolutamide showing promise for overall survival. Discussions are ongoing about which patients with mHSPC should receive this triple therapy approach. Christian Gratzke, Department of Urology, University Hospital Freiburg, Germany followed by describing how treatment decisions are made, including the role of imaging, with a case study of a patient with non-metastatic castration-resistant prostate cancer (nmCRPC), and another with mHSPC. A further panel discussion considered treatment options for various presentations of prostate cancer, and why one would be chosen over another. The panel concluded with a question and answer sessions that focused on when and why patients with prostate cancer are sent for genetic testing.
![]()
Erratum: Advancing Patient Care in the Evolving Prostate Cancer Treatment Landscape
Symposium speakers: Bertrand Tombal, Martin Bögemann, and Christian Gratzke
Original citation: EMJ Urol. 2022;10[1]:38-47. DOI/10.33590/emjurol/10110667. https://doi.org/10.33590/emjurol/10110667.
Date correction published: 23.08.22
In the article reviewing a symposium session presented at EAU22 by speakers Bertrand Tombal, Martin Bögemann, and Christian Gratzke in the August issue of EMJ Urology 10.1 the following sentence is incorrect, “We all would like to extend survival and delay progression [from localised prostate cancer] to nmCRPC.” The sentence should read, “We all would like to extend survival and delay progression [from localised prostate cancer] to mCRPC.”
EMJ apologises for the error and any inconvenience caused.
![]()
Non-metastatic Castration-Resistant Prostate Cancer: Improving Overall Survival and Time to Metastasis While Maintaining Quality of Life
Martin Bögemann
Prostate cancer varies at initial diagnosis and in its progression, with non-metastatic and metastatic presentations that may or may not respond to ADT (Figure 1).1 A major concern for doctors and their patients is the development of metastases, which affects QoL and OS. Although 90% of prostate cancer cases present with localised disease at initial diagnosis, failure to respond to ADT, known as castration resistance, will develop in 10‒20% of males with prostate cancer within 5 years of follow-up.2,3 The majority (86%) of patients with metastatic castration-resistant prostate cancer (mCRPC) will have progressed from nmCRPC.4
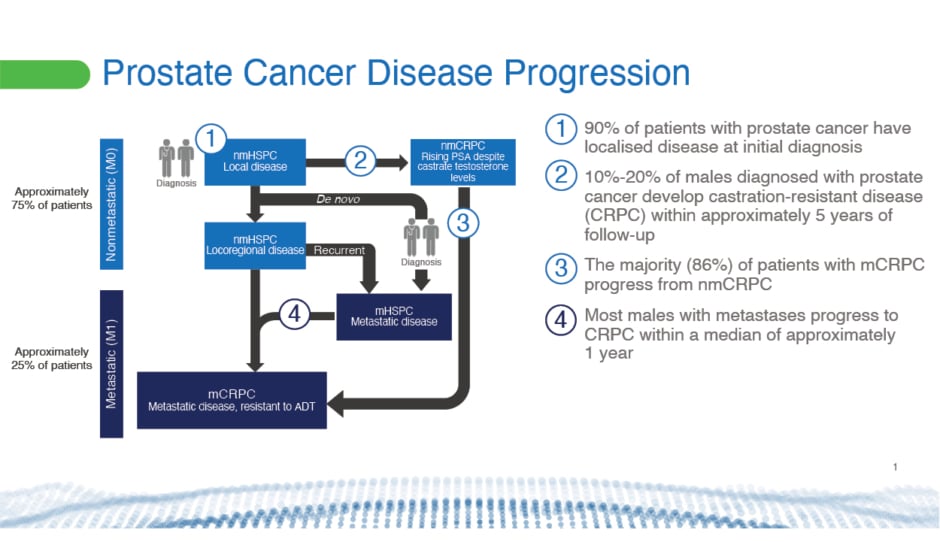
Figure 1: Prostate cancer disease progression.1-5
ADT: androgen deprivation therapy; M0: cancer has not spread to other parts of the body; M1: cancer has spread to other parts of the body; mCRPC: metastatic castration-resistant prostate cancer; mHSPC: metastatic hormone-sensitive prostate cancer; nmCRPC: non-metastatic castration-resistant prostate cancer; nmHSPC: non-metastatic hormone-sensitive pancreatic cancer.
The risk of annual all-cause mortality increases following progression from nmCRPC (16%) to mCRPC (56%).4 Five-year survival also falls after development of bone metastases from 56% to 3%.6 Bone metastases significantly reduce a patient’s QoL and increase healthcare costs.6-11 “It’s very important to keep patients away from metastasis,” said Bögemann.
Bögemann’s talk focused on treatment options for nmCRPC that can improve OS and time to mCRPC, while maintaining patients’ QoL.
One thing clinicians look for in their patients with nmCRPC is the time it takes for the blood levels of a biomarker called prostate-specific antigen (PSA) to double, known as the PSA doubling time (PSADT). The faster PSA levels double in the blood, the greater the risk for developing distant metastases, and the more urgent it is for them to receive treatment.12
Currently, three ARis are used to treat patients with nmCRPC. Enzalutamide and apalutamide have very similar molecular structures. Darolutamide, on the other hand, has a unique structure that increases its flexibility, and confers higher polarity and more hydrogen-bond-forming potential.13-17 Its structure could be the reason for its limited potential for drug-drug interactions (DDI), an important factor when managing comorbidities in patients with prostate cancer.18-20 The unique structure of darolutamide could also explain the drug’s low blood-brain barrier penetration in rodent models compared with other ARis.13-17,21 This finding was further supported in a study on healthy volunteers, in which darolutamide caused similar changes to placebo in regional cerebral blood flow.22 This could lead to a low potential for central nervous system-related adverse events in patients.15-17,21,22
The three ARis have been studied in three placebo-controlled Phase III trials.18,23,24 The ARAMIS trial (NCT02200614),25 sponsored by Bayer, studied the efficacy and safety of darolutamide in males with high-risk nmCRPC.19,26 The PROSPER trial (NCT02003924),27 sponsored by Pfizer, studied enzalutamide, and the SPARTAN trial (NCT01946204),28 sponsored by Aragon Pharmaceuticals, Inc., studied apalutamide.29,30 Each trial enrolled around 1,500 patients with nmCRPC with short PSA doubling times, equal to or less than 10 months.
Compared with placebo, all three ARis demonstrated significantly extended OS, reduced risk of dying (darolutamide by 31%; enzalutamide by 27%; apalutamide by 22%), and longer median times to metastasis.18,19,23,24,26,29,30
“Of course, those endpoints are not the only thing important for patients,” explained Bögemann. Softer targets, like time to cytotoxic chemotherapy or antineoplastic therapy, were also significantly improved with the three ARis.19,29,30
Importantly, patients treated with darolutamide showed a robust PSA response, which can help to reduce the PSA-related anxiety that affects many males with prostate cancer.31
To test drug tolerability, researchers consider drug discontinuation rates, incidences of adverse events, and deterioration of QoL. Darolutamide fared well overall, with a discontinuation rate close to that of placebo (8.9% versus 8.7%, respectively). It also had a very low incidence rate of side effects. For example, fatigue was reported in 33% and 46% of patients who were drug-treated in the SPARTAN and PROSPER trials, respectively, but in only 13% of patients treated with darolutamide in the ARAMIS trial.18,19,29,30 Patients treated with darolutamide reported a significant delay in deteriorating QoL scores compared with placebo.18,19,30 QoL was also maintained in patients who received enzalutamide in the PROSPER trial and apalutamide in the SPARTAN trial.29,30
Bögemann concluded that darolutamide can be used to extend the OS of nmCRPC patients with very good tolerability, helping to maintain QoL and alleviate PSA-related anxiety. “I think using darolutamide is very useful for the nmCRPC patients that we treat,” he added.
Metastatic Hormone-Sensitive Prostate Cancer: Does Early Treatment Intensification Improve Survival and Delay Progression to Metastatic Castration-Resistant Prostate Cancer?
Bertrand Tombal
mHSPC is prostate cancer that has metastasised but remains susceptible to treatment with ADT. It can be discovered at initial presentation of prostate cancer, or develop from recurring locoregional nmHSPC. Most males with metastases progress to mCRPC within a median of 1 year.5 Treatment guidelines have been modified for patients with mHSPC over the years, following the results of several clinical trials.
For years, said Tombal, hormone therapy and sometimes surgical castration were the only available options for treating patients with newly-diagnosed metastatic disease. Everything changed with the publication of the GETUG-AFU15 (NCT00104715)32 and CHAARTED (NCT00309985)33 trials (Figure 2), he said.34,35
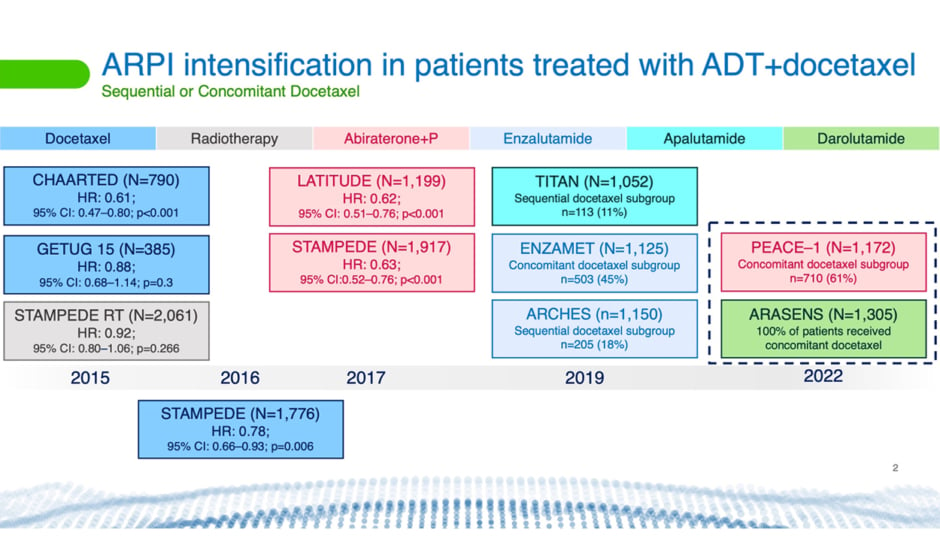
Figure 2: Timeline showing Phase III clinical trials using different therapies.5,34-41
ADT: androgen deprivation therapy; ARPI: androgen receptor pathway inhibition; CI: confidence interval; HR: hazard ratio; P: prednisone.
These trials, which ran from 2004–2008 and 2006–2012, respectively, assessed the efficacy and tolerability of ADT with or without docetaxel. This regimen was tested in males with de novo metastatic disease, known as synchronous metastases, and in males whose metastases developed following treatment of localised prostate cancer, called metachronous metastases. Both studies reported improvement in progression-free survival with ADT plus docetaxel compared with ADT alone. However, the GETUG-AFU15 trial did not find a significant difference between the two approaches in OS.
“So, for a few months, we lived with some uncertainty,” said Tombal. “And many times when it’s like this, you need a third trial to adjudicate.”
This is what the STAMPEDE trial (NCT00268476)42 provided (Figure 2).36 This trial, which has been ongoing since 2005, showed that adding docetaxel to ADT significantly increased OS in patients with mHSPC.
“If you ask me, 15 years down the line, what was the benefit of docetaxel?” said Tombal, “I would say that, beyond increasing OS, it was a tipping point for multidisciplinary management of prostate cancer. For the first time, urologists had to sit down with medical oncologists to discuss the best treatment for patients.”
The results also changed treatment guidelines. Clinicians were advised to give ADT in combination with chemotherapy to every patient presenting with stage M1 disease. “We have learned a lot since then,” added Tombal.
For example, a meta-analysis by the STOPCAP M1 collaboration of individual participant data from the CHAARTED,33 GETUG-AFU15,32 and STAMPEDE42 trials found that docetaxel appeared to improve progression-free and OS for all males with mHSPC, except those with low-volume, metachronous disease.43
The same study did show, however, that there was some benefit to giving docetaxel to patients with low- and high-volume stage T4 lesions. “But clearly, when it comes to docetaxel, volume and timing matters,” said Tombal.
Things changed once again for mHSPC treatment with the arrival of ARPIs. Currently, these are the three ARis, apalutamide, enzalutamide, and darolutamide, in addition to abiraterone acetate. “Within 5 years, we had confirmation from five trials (Figure 2) that adding an ARPI to ADT very significantly increased OS [versus ADT alone], notwithstanding volume and timing of metastases,” said Tombal.5,37-39,41,44
Current European Association of Urology (EAU) guidelines now recognise this. The guidelines recommend offering ADT in combination with docetaxel if first presentation is M1 and the patient is fit for treatment with the chemotherapeutic.45 ADT and abiraterone acetate plus prednisone, or apalutamide, or enzalutamide, are recommended for patients whose first presentation is M1 disease, and who are fit for that regimen.45 ADT and radiotherapy should be offered when first presentation is low-volume M1 disease.45 “[The guidelines] have not been reviewed yet for darolutamide, explaining why it is not there,” said Tombal. This may change with emerging evidence.
Now, two clinical trials, PEACE-1 (NCT01957436)46 and ARASENS (NCT02799602)47 (Figure 2), are investigating the effects of treating patients with mHSPC with a combination of ADT, docetaxel, and an ARPI.48,49
“When we designed the [ARASENS]47 trial with Bayer in 2014–2015, the question we asked was: if a patient requires ADT plus docetaxel, is there room to add one ARPI? For us, from the start, darolutamide was an optimal agent, because it had very little drug-drug interaction […] and was extremely well tolerated. We also knew from 22 trials in CRPC that docetaxel was a very difficult bride to be married,” because combining drugs with docetaxel often increases its toxicity.
The ARASENS47 trial, which evaluated concomitant ADT, docetaxel, and darolutamide in 1,305 patients with mHSPC, found a significant improvement in OS, with a 32.5% reduction in the risk of death compared to ADT and docetaxel alone.50 “Keep in mind that these were probably high-volume, high-risk patients with aggressive disease because they were selected [to receive] docetaxel,” said Tombal.
The trial found that addition of darolutamide did not affect patients’ ability to complete six full cycles of docetaxel, nor did it increase the incidence of adverse events or drug discontinuation due to treatment-emergent adverse events (Figure 3).50 Importantly, docetaxel toxicity did not increase.49 Docetaxel doses have to be reduced in some concurrent trials with other ARPIs to make it tolerable, said Tombal. “This is not the case here.”
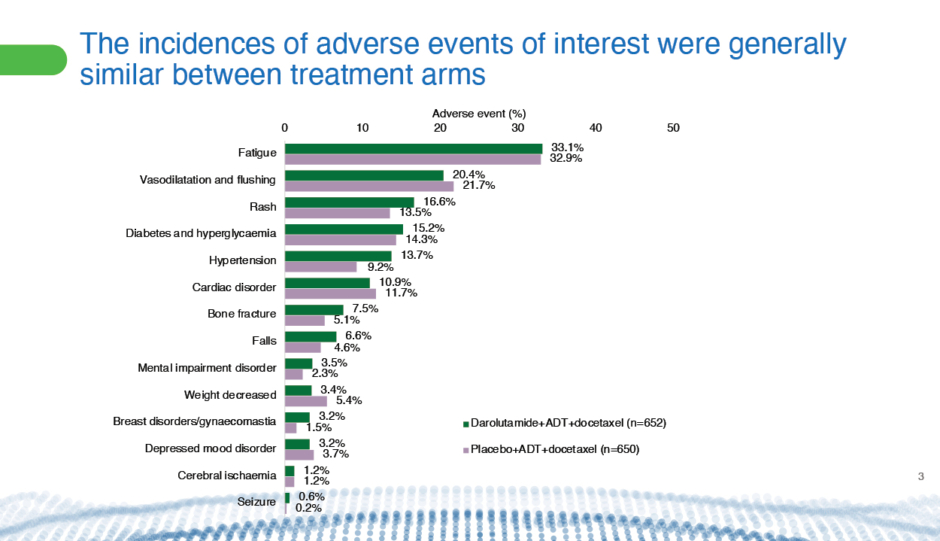
Figure 3: Comparison from the ARASENS47 trial of incidences of adverse events of interest between patients with mHSPC who received darolutamide plus ADT and docetaxel or placebo plus ADT and docetaxel.50
ADT: androgen deprivation therapy; mHSPC: metastatic hormone-sensitive prostate cancer.
Currently, discussions are ongoing about which patients should receive the triple therapy of ADT, docetaxel, and darolutamide.
Tombal and his colleague, Nicholas James, Professor of Clinical Oncology, Institute of Cancer and Genomic Sciences, University of Birmingham, UK, surveyed more than 70 participants at the Advanced Prostate Cancer Consensus Conference (APCCC) in 2021 about their preferred treatment options for the management of newly-diagnosed mHSPC. The survey found that 40% of participants recommend treating synchronous, high-volume mHSPC with the triple therapy of ADT plus docetaxel and ARPI.51
The question then remains: which of the ARPIs should be used in combination treatment of mHSPC? Comparing them can be tricky, explained Tombal, as different drug trials have used different patient populations and measured side effects differently. “So you have to be extra careful.”
A simple metric to look for, in this case, is the proportion of patients who discontinue treatment due to adverse events. The ARASENS47 trial showed a percentage difference of 3.0% between patients with mHSPC who discontinued combination treatment of darolutamide, ADT, and docetaxel compared with the arm given ADT, docetaxel, and a placebo.50 On the other hand, in the PEACE-1 trial, there was a 16% difference between the two arms of those treated with ADT and docetaxel plus with abiraterone and prednisone or ADT and docetaxel plus a placebo.48
“Abiraterone is a little bit more complicated to use compared with the other ARPIs apalutamide, enzalutamide, and darolutamide,” said Tombal. This is because patients treated with abiraterone need frequent monitoring for side effects and DDIs.52 This is especially important for patients with prostate cancer receiving treatments for comorbidities, which can often be the case.53,20
The take-home messages, said Tombal, are that docetaxel, abiraterone, and enzalutamide were first established as the standard of care for patients with mCRPC. The EAU guidelines then moved to recommend immediate intensification of ADT with an ARPI for treating patients with mHSPC.45 Additional intensification with docetaxel should be discussed, said Tombal. Importantly, the DDI profile of darolutamide differs from that of other ARPIs, which is relevant to patients who are polymedicated. Finally, a favourable toxicity profile, convenient management, and different DDI profiles should be considerations in the treatment of patients with prostate cancer, concluded Tombal.
Case Studies in Non-metastatic Castration-Resistant Prostate Cancer and Metastatic Hormone-Sensitive Prostate Cancer: Translating Data to Practice
Christian Gratzke
“We all share the same treatment goals for males with nmCRPC and mHSPC,” began Gratzke. “We all would like to extend survival and delay progression [from localised prostate cancer] to mCRPC. We would like to minimise adverse events […] and also preserve long-term quality of life. I think quality of life preservation has now become a major issue. Patient-reported outcomes are more and more important, and I think are a key criterion for judging the success of a certain drug.”
Gratzke described two case studies from his professional practice to illustrate how clinicians approach the treatment of different patients with prostate cancer.
A 44-Year-Old Patient with Metastatic Hormone-Sensitive Prostate Cancer
In July 2020, the PSA level of an asymptomatic 44-year-old male, whose father was recently diagnosed with prostate cancer, was found to be surprisingly high (18.2 ng/mL). They were given a 3-day course of antibiotics to rule out infection, and the test was repeated a month later. However, the PSA level had increased to 19.0 ng/mL. Imaging and biopsies suggested high suspicion for malignancy and metastasis.
Following discussions with a multidisciplinary team, the patient opted for a radical prostatectomy with extended lymphadenectomy in August 2020. Histology results from the resected tissue confirmed the previous findings. Eight weeks after surgery, the patient’s PSA level had dropped from 19.00 to 0.71 ng/mL. Further imaging suggested a malignant lymph node in the right side of the iliac fossa. The patient was started on ADT and radiotherapy to the prostate bed and affected lymph nodes. The PSA level fell to 0.04 ng/mL, but rose again 2 months later in April 2021 to 0.06 ng/mL. By August 2021, the PSA level had reached 0.22 ng/mL.
The patient opted for a salvage lymphadenectomy to remove the malignant lymph node, but their PSA level rose to 0.24 ng/mL 2 months later. Subsequent imaging and biopsy in March 2022 revealed new onset of liver metastases. The patient was placed on ADT, docetaxel, and darolutamide according to the ARASENS47 protocol and, as of June 2022, has completed three full cycles. The patient has lost their hair and developed lymphoedema in the left leg, with thrombosis ruled out, but is otherwise doing well. The patient is determined to complete all six cycles of the triple therapy with the expectation of a good QoL once they are off chemotherapy, and is receiving only ADT and darolutamide.
A 68-Year-Old Patient with Non-metastatic Castration-Resistant Prostate Cancer
A 68-year-old male with no family history of cancer, and who had PSA levels within normal range for 10 years during regular checkups, suddenly presented with a PSA level of 6.55 ng/mL. The patient was given a diagnosis of high-risk, locally advanced prostate cancer following imaging and biopsy. They also had previously diagnosed non-valvular atrial fibrillation and hyperlipidaemia, and were receiving medication for these conditions.
Histology results following a radical prostatectomy and lymphadenectomy confirmed prostate cancer Grade T3a, International Society of Urological Pathology (ISUP) 4, with negative margins, and no positive lymph nodes.
The patient recovered well but, 2 years later, in March 2017, their PSA level rose to 0.25 ng/mL, so they received radiotherapy and ADT. By May 2017, the patient’s PSA levels had dropped to 0 ng/mL.
In December 2018, the patient’s PSA level had risen again above 2 ng/mL and their testosterone fell significantly to reach castrate levels. Their PSADT was around 2 months, and a CT scan was negative for metastases but positive for local recurrence, including in small lymph nodes in the pelvis.
Following proactive discussions of the options with oncologists, the patient was given darolutamide according to the ARAMIS25 protocol. Scans carried out 16 weeks later showed that the pelvic lesions were progressively shrinking and no new lesions were apparent. The patient continues to do well on darolutamide and is experiencing only minor fatigue. They are waiting for their next imaging follow-up.
Discussion
Imaging and the Treatment of Non-metastatic Castration-Resistant Prostate Cancer
Tombal began the panel discussion by asking about the role of modern imaging in treatment decisions for patients with nmCRPC.
Gratzke and Bögemann agreed that a negative prostate-specific membrane antigen (PSMA) PET scan is not a reason to delay ARi treatment in a patient with nmCRPC who is in “good shape,” but shows a PSADT of 7 months.
“Why would you wait if you had a drug that is able to prolong survival and metastasis-free survival with a good quality of life?” said Gratzke. “There is no real, good argument to do so.”
A PSADT of 7 months means there is a high risk of developing metastasis within a short period of time, added Bögemann. So, even if the PSMA PET scan is negative, why wait, he agreed.
Tombal provided another example of a patient who had undergone prostatectomy and radiotherapy following rapid progression. Initially, their PSMA PET scan was negative and so ARi treatment was deferred. However, 3 months later, their PSA had doubled and a subsequent PSMA PET scan showed a large metastasis on the hip. The patient’s doctors opted for radiation therapy only. Tombal asked if the panel agreed with this approach.
When there is systemic disease, there is a very good rationale for offering systemic treatment, Gatzke responded. Radiation is still important, however, especially if the patient is experiencing pain or has a risk of fracture.
However, Gatzke explained that another patient described by Tombal with a very slow PSADT of 1 year and a PSMA PET scan with two defined bony metastases would be considered low risk. In this case, radiation therapy would be used to treat the metastases, with frequent imaging to monitor the response. In contrast to the previous patient with a very short PSADT, it is acceptable to delay systemic treatment in this low-risk case until imaging shows further progression, Gatzke said.
Triple Treatment Options for Metastatic Hormone-Sensitive Prostate Cancer
The panel then discussed options for triple therapy treatment of patients with mHSPC. High-volume mHSPC patients are considered good candidates for this approach, whereas low-volume, recurrent patients are not. The choice of ARPI to use within the triple therapy regimen should be guided by currently available evidence, which points, said Bögemann, towards darolutamide and abiraterone. Gratzke added: “Even if this was not an industry-sponsored symposium, I would not know why I wouldn’t be prescribing darolutamide in that aspect.”
Is There a Rationale for Genetic Workups Before Treatment?
Panellists answered this question from the floor, with Tombal explaining that current guidelines advise genetic workup in patients with a family history of cancer, with newly-diagnosed metastatic cancer, and in patients with intraductal and cribriform prostate cancer.
Patients with newly-diagnosed metastatic cancer found to have certain genetic mutations could qualify for treatment with a class of drugs called poly (ADP-ribose) polymerase inhibitors that cause cell death. Otherwise, genetic workups are more for the purposes of genetic counselling, and have broader implications for research, explained Tombal.








