Abstract
Ureteral fistulas are a rare occurrence that can arise from iatrogenic trauma, radiation, malignancy, and inflammation. Treatment options of urinary tract fistulas are handled on a case-by-case basis, and can necessitate a surgical approach. The authors present the case of an 85-year-old female patient with a ureterocutaneous fistula, where conservative management with percutaneous nephrostomy is a viable alternative to surgical intervention.
Key Points
1. This article describes the approach in management of a fistula for a patient unwilling to undergo surgery. It offers serial percutaneous nephrostomy catheter exchange as a viable surgery alternative for patients with ureterocutaneous fistulas, who are otherwise unwilling, or unable, to undergo surgery.2. The importance of this article is highlighted in the rarity of its presentation, as well as its management. Ureterocutaneous fistulas, as presented in this case report, are not common occurrences, and management can require surgery.
3. The authors hope that clinicians will accept their approach as a possible method of management for these types of fistulas. They also recommend that clinicians continue to take an individualised approach with fistulas, as every case is unique, and requires special considerations.
INTRODUCTION
Ureteral fistulas are a rare occurrence, and are most commonly the result of iatrogenic trauma, although other causes include radiation, malignancy, and inflammation.1,2 Treatment of urinary tract fistulas often depends on their severity and location, with some fistulas requiring conservative therapy, whereas surgical repair can also be utilised in refractory cases.3 Multiple complications can result from fistulas, such as electrolyte imbalances, and commonly hyperchloremic metabolic acidosis with hypokalaemia in the setting of enterovesical fistula.4 Elevated creatinine and uraemia with normal kidney function can also occur secondary to fistula, due to the reabsorption of these products.5
Radiation fistulas are frequently difficult to manage, as intervention must be performed after a 3–6-month period to allow for the greatest success. If treated prematurely, there is a risk that the inflammation in the affected areas has not diminished.1 Iatrogenic trauma has also been cited as a potential cause for ureteral fistula development in patients.6 Conservative measures are the first line of treatment for uncomplicated ureteral fistulas, including watchful waiting before moving on to ureteral stent, or percutaneous nephroureteral stent placement. When necessary, surgical options include the excision of fistula with anastomosis or grafting.1
Ureterocutaneous fistulas are rare, and seldom discussed in literature. Reported here is a case of ureterogluteal fistula with concurrent presacral abscess secondary to previous pelvic radiation history and potential iatrogenic trauma.
CASE PRESENTATION
An 85-year-old female presented to the emergency department (ED) with vaginal pain, subjective fevers, and clear discharge from her left buttock for 2 days’ duration. The patient’s past medical history included a known draining, presacral abscess involving the left gluteal muscle, and Stage IIb cervical cancer diagnosed 11 years prior to initial presentation that was treated with cisplatin and radiation. Additionally, this was complicated by retroperitoneal fibrosis causing extrinsic compression of bilateral ureters, requiring bilateral nephroureteral stents. The patient also had a history of chronic urinary tract infections (UTI) with extended spectrum β-lactamases Klebsiella. On presentation, the patient’s blood pressure was 112/58 mmHg, pulse of 80 beats/minute, respiratory rate of 16 breaths/minute, a temperature of 37 °C, and oxygen saturation of 98%. The patient’s creatinine was 1.71 mg/dL, and she had a white blood cell count of 5.7×103 uL, and a lactate of 3.5 mg/dL. Urinalysis displayed 3+ protein, 4+ blood, positive nitrites, 4+ leukocytes, and many bacteria, suggestive of UTI. A CT scan of the abdomen and pelvis without contrast was performed, revealing a 6.0×5.4×3.0 cm presacral abscess involving the left gluteus maximus muscle, with fistulous communication to the skin surface, in addition to bilateral severe hydronephrosis (Figure 1). In the ED, the patient was started on empiric vancomycin and aztreonam, and admitted for gluteal abscess with extension and acute kidney injury.
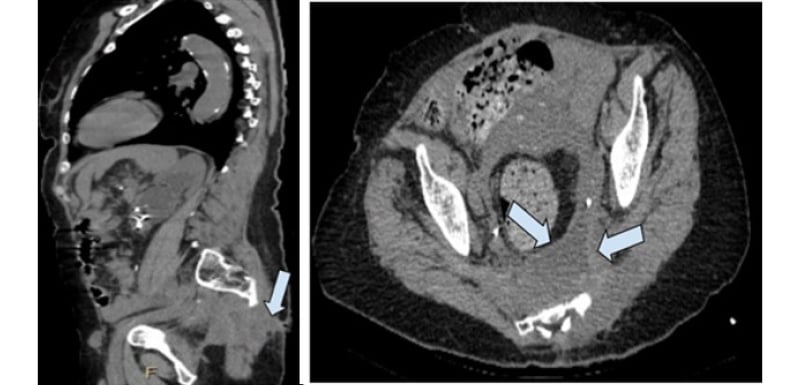
Figure 1: CT of the abdomen and pelvis.
CTAP is without contrast in transverse plane (left panel) showing a 6.0×5.4×3.0 cm presacral abscess
(arrow) involving the left gluteus maximus muscle. Sagittal plane CTAP without contrast (right panel)
displaying presacral abscess with fistulous communication to the skin (arrow).
CTAP: CT of the abdomen and pelvis.
During the patient’s admission, wound fluid creatinine measured 55.00 mg/dL, with serum creatinine 1.16 mg/dL, and lactate 1.80 mg/dL, leading to suspicion of a ureteral fistula. As the gluteal abscess was draining clear fluid on presentation, General Surgery recommended extending the drainage site to improve the abscess; however, the patient refused intervention initially. Urine cultures also revealed Klebsiella pneumonia, requiring initiation of ceftazidime and avibactam per Infectious Disease. The Klebsiella infection was believed to be due to a suspected fistula. As a result of the continued infection, General Surgery deferred to Urology and Interventional Radiology. A cystoscopy with bilateral retrograde pyelogram for bilateral nephroureteral stent exchange was performed per the patient’s extensive history of UTIs and chronic stents. A cystogram and retrograde ureterogram were also performed, but did not reveal any ureteral or bladder extravasation indicative of a fistula at the time. During this time, the patient was continued on meropenem for management of the gluteal abscess and gram-negative UTI. On Day 6 in the hospital, interventional radiology performed a CT-guided aspiration and catheter placement of the left presacral collection. The CT-guided aspiration was delayed, as the patient was not agreeable until then to any drainage or incision, despite advice from the managing team. Cultures from the collected abscess also showed Klebsiella pneumonia, along with Candida glabrata, for which anidulafungin was added.
On Day 9 in the hospital, the patient’s presacral drain was exchanged by Interventional Radiology due to dislodgement. During exchange, contrast was seen collecting in the presacral space, as well as the distal left ureter and urinary bladder, suggestive of a fistulous tract with the distal left ureter (Figure 2). Due to this fistulous tract, the patient’s left nephroureteral stent was removed, and converted to a left percutaneous nephrostomy (PCN) catheter. This was done to decrease drainage output to the affected area, and promote healing. The patient was ultimately discharged with a left PCN and gluteal drain, due to the refusal to undergo any further invasive interventions; continued high output; and instructed to follow-up with Urology as an outpatient.
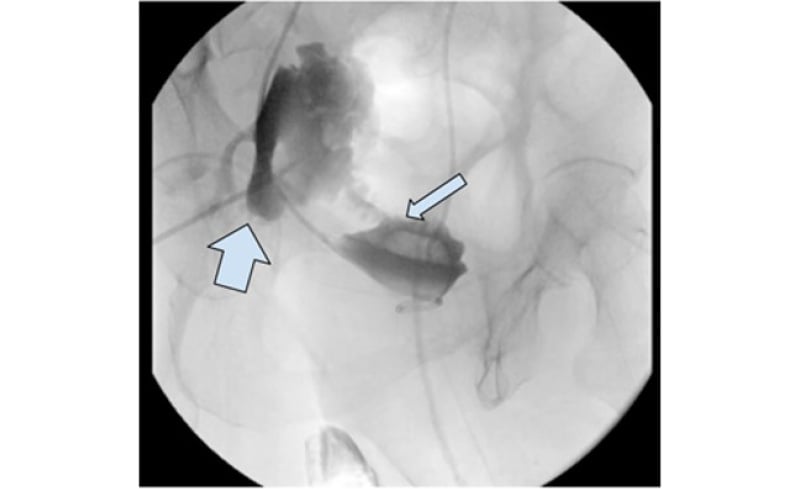
Figure 2: Interventional radiology study of injected contrast into the presacral region.
This reveals the collection of contrast in presacral space (thick arrow), as well as collection within the left distal ureter (thin arrow) and urinary bladder, suggestive of a fistulous tract between the ureter and presacral space.
Approximately 9 months after initial presentation, the patient returned to the ED with a chief complaint of PCN displacement. The patient mentioned she had experienced some drainage from the initial gluteal site, and had mild left flank pain. Urinalysis revealed no bacteria growth, creatinine was 0.82 mg/dL, and the patient had a white blood cell count of 7.8×103 uL. On examination, no fluid was identified in the gluteal abscess drain. CT abdomen and pelvis without contrast showed resolution of bilateral hydronephrosis, and the fluid collection previously found in the left presacral space was no longer present. An antegrade nephrostogram showed extravasation to a gluteal abscess drain, revealing a persistent presacral abscess and fistula of the left ureter (Figure 3). The patient was instructed to undergo routine nephrostomy catheter exchange in 4–6 weeks as an outpatient to manage the persistent fistula of the left ureter.
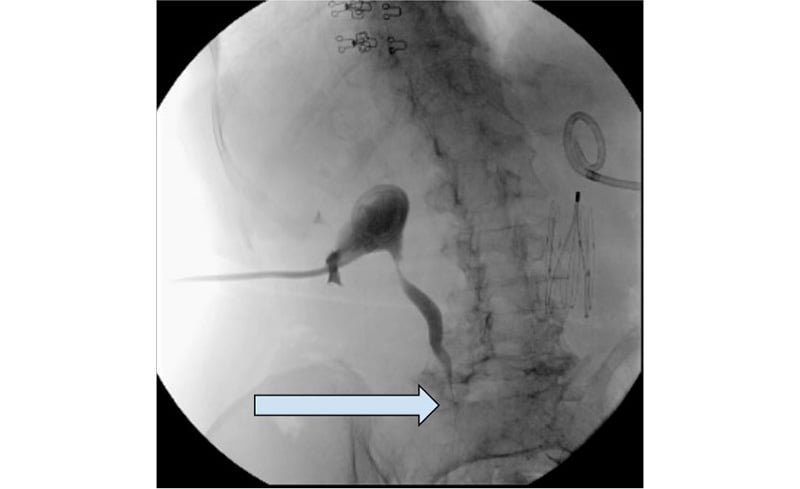
Figure 3: Antegrade nephrostogram showing contrast extravasation at the level just below the iliac crest to the abscess.
DISCUSSION
Ureterocutaneous fistulas are rare, and while iatrogenic is most common, cases involving non-surgical trauma, calculous disease, and granulomatous inflammation have been reported.7 Due to the often post-surgical aetiology, ureteral injuries are diagnosed with delay, and usually only become apparent once the patient becomes symptomatic.8 Continuous drainage from the fistula can be seen in patients with ureterocutaneous fistulas similar to the described case.9 If a ureterocutaneous fistula is suspected, fluid and serum creatinine levels should be obtained. If the creatinine level in the fluid is at least 18% or more than the serum creatinine levels, this potentially signifies urinary leak.10 A urologic CT and fistulography can be used as an imaging modality for fistula diagnosis.9
The fistula in this case was most likely a complication of several processes, including the inflamed collection, prior radiation, stent placement, and gluteal abscess drain placement. Prior radiation for cervical cancer in this patient possibly induced obliterative endarteritis, leading to tissue hypoxia, denervation, and fibrosis of the tissue. The vascular insult to the ureter caused mucosal breakdown, which weakened the area, making it susceptible to the initial formation of the fistula.11,12 The fistula resulted from the chronic inflammatory state, concomitant with a presacral abscess at presentation, and the radiation-induced tissue injury. These issues, in tandem, prevented the fistula from resolving. A ureterocutaneous fistula is rare in nature, as radiation is a risk factor, and unique in the structures that it affects. Radiation, however, can induce many other rare types of fistulas, including ureteroarterial fistulas and ureterovaginal fistulas.
Nearly half of fistulas can be attributed to radiation. Previous literature reports 0.3–3.0% are secondary to brachytherapy, 0.6% are related to external beam radiotherapy, and 2.9% are related to combination therapy.13 There is no current evidence that states that radiation-induced fistulas differ in outcomes when compared to abscess-induced fistulas. However, of the urinary complications that occur from radiation therapy, urinary fistulas are the most difficult to manage, and often require surgical intervention.12 The treatment of ureterocutaneous fistulas is well-described in literature. Conservative management can lead to the spontaneous resolution of fistulae in 35.7% of patients.14 Similarly to the treatment of fistulas and abscesses in general, intervention aims to divert the urine away from the fistula, and promote abscess healing. Nephroureteral stent placement is the first preferred method of treatment for ureteral fistulas, with a success rate of 63.0% for patients with uterovaginal fistulas.15 If access cannot be obtained, percutaneous nephrostomy tube placement is often employed for urinary diversion. Surgical excision is also used in the case of ureterocutaneous fistulas, to close off the affected area. If the patient was amenable to surgery in this case, the team would have first visualised the bladder and ureters with a cystoscopy. A stent would then have been inserted to the level of the fistula. Afterwards, a fistulectomy would be attempted, with surgical excision of the fistula. A waterproofing interposition layer would be placed, before finally closing the area. In providing a waterproofing layer, the opening to the skin is closed off, minimising recurrent infections. Alternative surgical approaches include simple stitches to close the area without the introduction of an interposition layer. This approach has greater efficacy in smaller fistulas. In larger fistulas with unhealthy skin, waterproofing provides the best benefit. Occasionally, larger fistulas require possible ureteric reconstruction with stent placement.16 A systematic review demonstrated that any type of waterproofing technique used to close a fistula of the urinary tract has extremely high success rates.17
The major takeaway from this case report is that conservative PCN replacement is a viable treatment method for patients with a ureterocutaneous fistula who are unwilling to undergo procedures. It is a suitable method that can show adequate management of this specific issue when surgery is not achievable. This can be applied in future cases where patients are not amenable to surgery, or if there are significant comorbidities that make surgery an impractical option. However, as these cases are rare, and often involve prior iatrogenic trauma, the authors find that management must be personalised for each patient.
CONCLUSION
Presented here is the first reported case of a ureterocutaneous fistula with gluteal abscess formation, in a female with prior retroperitoneal radiation and subsequent fibrosis treated solely with a PCN tube. After the discovery of fistula formation to a gluteal abscess, serial PCN exchange, carried out approximately every 4–6 weeks, has been used to mitigate urine flow through the fistula. This has allowed for a resolution of the patient’s gluteal abscess, and decreased drainage through the sinus tract 9 months after initial presentation.







