Abstract
Since the recognition of asymptomatic bacteriuria (ABU), several studies have questioned its significance. It is a very common condition, observed in many healthy individuals. Current guidelines mandate that ABU should not be treated in all cases, as it does not seem to improve the outcome. Conditional restrictions for treatment of ABU can be relaxed in certain situations, with minimal exceptions to the rule.
INTRODUCTION
The diagnosis of urinary tract infections (UTI) has a major role in its subsequent management. UTI are one of the most common infections reported in any hospital. Antibiotics are prescribed for most infections. Therefore, it is important to note that more often than not antibiotics are overprescribed, as is the case in the treatment of asymptomatic bacteriuria (ABU).1 This short review will analyse the significance of ABU in adults with reference to the current guidelines and recommendations.
The role of the microbiology laboratory in the management of UTI is to support the clinical care through early, accurate diagnosis, with appropriate antimicrobial sensitivity testing. To date, the gold standard laboratory test for the diagnosis of UTI has been culture. The concept of ABU arose from the seminal work of Edward Kass,2-4 who used criteria for assessing the significant bacterial count. A count of ≥105 colony forming units (CFU) of bacteria per mL of urine was considered to be significant and to distinguish between the true pathogen and the indigenous flora. Later studies have shown that colony counts less than this can also be reflective of a true UTI.5-7 Therefore, it is time we examined the reliability and applicability of this age-old concept in the laboratory diagnosis of UTI.
Current guidelines recommend us not to treat ABU, except in pregnancy and prior to most urologic surgeries where mucosal bleed is expected.1,8 In a catheterised patient, it is difficult to determine whether bacteriuria signifies asymptomatic colonisation of the catheter or true infection. Most often the signs and symptoms of UTI are nonspecific or potentially attributable to another source of infection in these patients. It is noteworthy that in both non-catheterised and catheterised patients, the presence of pyuria is often considered an important finding.1,9,10 Therefore, a combination of urinalysis and clinical presentation is needed to diagnose UTI.
PREVALENCE OF ASYMPTOMATIC BACTERIURIA
The term ABU refers to the isolation of bacteria in significant counts (>100,000 bacterial/mL) of a single bacterial species from a clean catch urine specimen of an individual who has no acute signs or symptoms. Though the detection of significant counts of bacteria in a clean catch midstream urine sample is practically acceptable, it has low credibility in women, unlike men, as the probability of a woman having true bacteriuria is only ˜80% with a single specimen. This probability increases to 95% if two or more consecutive cultures are positive for the same organism.4,11,12 In the absence of other reliable modes of diagnosis, quantitative culture remains the gold standard for diagnosis of ABU in pregnancy.13-15
ABU is fairly common in the general population amongst adults, and as documented earlier,10 is more common in women than in men, and increases with age.8,16,17 ABU has been documented in 1–5% of premenopausal healthy women, which subsequently increases with age. Sexually active women tend to have a five-times higher prevalence than women who are not sexually active.8,18,19 Compared to young women, ABU is uncommon in young men and, if present, signs of prostatitis should be looked for.8 The reported prevalence rates of ABU in pregnancy range from 2–15%.20 If left untreated, 20–30% of these may develop acute pyelonephritis by the second or third trimester.18,21-24 ABU, which is otherwise a benign condition, is a cause for concern during pregnancy by most clinicians. Due to the growing fetus, the enlarged uterus impinges on the bladder, leading to urinary stasis and ureters, which cause hydronephrosis. This is compounded by the smooth muscle-relaxing effect of progesterone, which dilates the ureteric sphincter allowing the reflux of the urine into the renal pelvis, resulting in pyelonephritis which may have an ominous effect on the maternal, as well as the fetal, outcome. Among several other factors contributing to this, the most notable are glycosuria (due to gestational diabetes) and proteinuria in pregnancy-induced hypertension, both of which promote bacterial growth. Bacterial products, namely endotoxins, can result in secretion of pro-inflammatory cytokines by either the maternal or fetal macrophages, leading to precipitation of labour.20-24 ABU in postmenopausal women varies from ˜5–19%, and is much more common in women with a prior history of UTI.8,18,25 There are many contributing factors, which include a diminishing oestrogenic effect on the genitourinary mucosa, urologic abnormalities such as cystoceles, gynaecological abnormalities like genitourinary prolapse, any surgical manipulations on the genitourinary tract, and genetic predisposition.26 In elderly men, the prevalence of ABU ranges from 4–7%; the major predisposing factor is prostatic enlargement leading to bladder outlet obstruction.27 With increasing age, dementia, and decreased mentation, impaired bladder voiding, and incontinence of the bladder and bowel, compound the existing structural and functional impairments, resulting in a higher frequency of bacteriuria.27 Contrary to some earlier studies,28,29 ABU in the elderly is not associated with increased mortality.10 The prevalence of ABU is also found to increase with associated co-morbidities, like diabetes mellitus.17,30-32 In individuals with spinal cord injury, there is incomplete bladder emptying, which promotes bacterial growth, thereby resulting in bacteriuria.31,33 ABU has been found to be higher in the early post-transplant period in cases of renal transplant recipients.31 Moreover, immunosuppressants can mask the signs and symptoms of infection, making distinguishing between ABU and a true infection difficult.8,31
PATHOGENESIS OF ASYMPTOMATIC BACTERIURIA AND THE ROLE OF MICROBIAL FLORA
There are many factors which can predispose an adult to having ABU, including the genetic composition of the host, the presence of a foreign body, incomplete bladder emptying, any prior instrumentation, etc. In the elderly, the reasons for a higher prevalence of ABU are multifactorial. Due to the reduced immune response in the elderly, this colonisation tends to be persistent and more so in diabetics, as documented earlier.34-38 Neurogenic bladder and resulting incontinence, obstructive uropathy, altered bladder mucosal defences and reduced effective cell-mediated immunity, alterations of urinary and vaginal pH as a result of declining hormonal secretions, and glycosuria, can result in increased colonisation, due to bacteria in the urogenital tract.17,34-38 Glycosuria encourages the growth of bacteria in vitro, but it is not well ascertained whether ABU is a precursor of symptomatic bacteriuria in patients with diabetes.39-41 ABU does not lead to severe complications in diabetes; hence, routine screening is not warranted in these individuals.42 Certain genetic polymorphisms in the innate immune receptors that downregulate the immune response, for example the TLR4 promoter region, have been linked to ABU state. Conversely, increased levels of interleukin (IL)-8 have been observed in individuals with ABU. This increase is, in turn, triggered by the binding of the fimbriae and the lipopolysaccharides to the specific TLR4.43,44 This activation causes recruitment of the neutrophils, which aids in localising the organism within the bladder and prevents it from ascending.
The commensal flora of the periurethral area, vagina, or the gut are usually the source of the bacteria isolated from the urine of patients with ABU. Most often (80–90%), the organism is Escherichia coli, followed by Enterococcus, Klebsiella, and Proteus. A variation in the culture isolation of the organisms depends on the patient characteristics; namely, diabetes, female sex, pregnancy, whether they are catheterised, old age, etc.45 The organism can exhibit many relationships with the host in various forms, either as a commensal, coloniser, or a pathogen. It is interesting to note that there are strains of E. coli which have been isolated from cases of ABU. Studies have documented that strains of E. coli colonising the urothelial cells in these patients are less virulent,46-49 the best studied being E. coli 83972, and those that have been isolated from symptomatic UTI cases are identified as uropathogenic E. coli (UPEC), the best characterised being UPEC CFT073. Conversely, UPEC strains have also been isolated from cases of ABU, as otherwise ABU strains could not be differentiated from those causing cystitis or pyelonephritis.50-52
Using comparative genomics, it was noted that E. coli 83972 and UPEC CFT073 were phylogenetically related and had similar origins. The former evolved from a pathogen to become a commensal, shedding its virulent characteristics. Certain point mutations have been detected in the gene expressing the papG fimbria (because of which the P fimbriae can no longer adhere to its receptor) and in foc D, which is located in the outer membrane, behaving like a usher protein for F1C fimbriae, thus rendering it incapable of reaching the cell surface.47,53 Moreover, this evolution is driven by host factors, which have been observed in many studies but are not well understood.54 Notably, there are many adhesins that aid in the colonisation of both UPEC and ABU strains of E. coli, but none act in isolation; it is always a combination of these adhesive factors that culminate in symptomatic infection or an ABU. Hence, there are many more factors that may be responsible in the variation of gene expression of this organism, and which are responsible for the outcome of the disease state of the host; either an ABU or a symptomatic infection.
SAMPLING OF URINE
The most practically feasible and routinely submitted sample for a culture and urinalysis for diagnosis of UTI is the midstream clean catch urine, which is collected early in the morning. The sample, if collected in this manner, is likely to give a clear picture of the contents of the urine, which in turn forms an approximate reflection of the ongoing processes in the urinary tract.55 Errors arise due to the improper cleaning of the skin and surrounding areas while collecting urine. The use of catheterisation to collect samples is not advisable either, as the skin contaminants may gain access to the urinary tract, which can be harmful, and the distal urethral microbial flora can be allowed to ascend, which can result in a UTI.56
In cases where the patient is catheterised, the sample can be easily obtained from the sampling port of the catheter after cleaning it using appropriate, routinely used, antiseptics. A suprapubic puncture is by far the ideal sample, as it is collected directly from the bladder under strict aseptic precautions,56,57 but, being an invasive procedure, is rarely opted for unless indicated.
The sample collected by any of the above means should be transported to the laboratory within 2 hours of collection or should be stored under refrigerated conditions (2–8°C) for a maximum of 24 hours. In cases of delay, some preservatives, such as boric acid (1.8%), sodium chloride-polyvinyl pyrrolidone, and boric acid-glycerol-sodium formate, can be used, but there are problems inherent to the use of such agents, as described previously.6,55-57
Considering the prevalence estimates of ABU in different groups, the recommendations for screening of these same groups applies with conditions. The screening may not be beneficial in certain groups where the prevalence is high and treatment does not have any effect on the outcome of ABU (Table 1).8,35,50,58
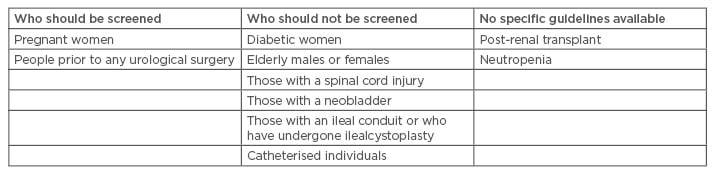
Table 1: Recommendations for screening of asymptomatic bacteriuria.
INTERPRETING URINALYSIS AND INTERPRETING URINE CULTURES
The significance of colony count varies depending on a number of factors, including the patient, effect of antimicrobial therapy, stage of infection, fluid intake/frequency of micturition, underlying illness, presence of a urinary catheter, age and sex, pH of urine, growth rates of the organisms, site of infection, and residual urine, etc.10,17 Growth of a single organism in significant counts is to be considered in cases of ABU in pregnancy and prior to urological surgeries, for the reasons stated above.10,17 Isolation of more than one organism on culture reflects contamination in a midstream clean catch urine to more accurately reflect the fact that co-infection or multi-species infections are uncommon but do occur given the overall burden of ABU, and therefore avoids misinterpretation. In general, two consecutive urine samples in women need to be analysed for the diagnosis of ABU.10 More often than not, in most of these cases, the follow-up sample may not match the initial positive culture result, which is indicative of a contaminated specimen. In the case of catheterised urine, a threshold of ≥1,000 CFU/mL is considered significant, though in many centres even lower CFU is considered significant.10 In such cases, the sampled organisms may be from biofilms on the inner surface of the catheter; hence, culture reports of catheterised urine may not accurately reflect bladder bacteriuria.
Pyuria is often reflective of an ongoing UTI, especially upper UTI, but it is not specific to UTI.9,59,60 The presence of pyuria is not necessarily linked to inflammation due to infection in the urinary tract. It has also been noted in healthy individuals, including schoolgirls, women with ABU, and in those with chronic indwelling catheters. Very often, individuals with ABU do not have pyuria, in which case the rapid tests to detect leucocyte esterase will be negative. Thus, pyuria has a limited role to play in the context of ABU; its presence or absence should not influence the use of antibiotics. Also, one needs to note that rapid dipstick tests to detect nitrite, as well as the leucocyte esterase, have several limitations of their own. Thus, they are not used for diagnosing ABU.50,61
ROLE OF TREATMENT
ABU is a common clinical finding. It may be observed in otherwise healthy individuals, as well as in those with functional or morphological defects of the genitourinary tract, the burden of ABU being higher in the latter. This creates a dilemma among clinicians and health professionals as to whether or not to treat this condition.
Guidelines strictly recommend screening and treatment of ABU in cases of pregnant women, to reduce chances of pyelonephritis and reduce associated fetal damage.10,17 The isolation of Streptococcus agalactiae from the urine of a pregnant woman also needs to be treated with antibiotics, because of the potential risk to the baby during vaginal delivery.45 Surprisingly, despite the large amount of literature available, there is little evidence to support the fact that treatment of ABU in pregnancy actually reduces the risk of preterm labour.8,50,60 Also, most of the existing guidelines do not mention the duration of therapy of ABU during pregnancy. Guidelines also recommend screening and treatment of ABU prior to urological interventions, as surgery causes mucosal injuries which allow these bacteria to penetrate the tissue, causing local infection, and can even gain access to the blood circulation resulting in bacteraemia.8 Studies have shown no benefit in treatment of ABU in patients with an ileal conduit, ileocystoplasty, or orthotopic neobladder, and patients using clean intermittent catheterisation, as they frequently become colonised and it is difficult to eradicate this colonised flora using antibiotics.8,50 Antibacterial treatment of ABU in diabetic patients failed to reduce the risk of symptomatic UTI and infectious complications, while untreated ABU did not correlate with any increase in complications. In such cases, it is important to understand that with good control of diabetes itself the risk for symptomatic UTI and infectious complications can be reduced.8,50
Cohort studies, as well as placebo-controlled trials, involving spinal cord injury patients have not shown any decrease in symptomatic infection when antibiotic therapy for ABU was used. Reports show that low urinary bladder pressure aids in preventing renal failure in the presence of ABU in such cases.61 Fiorante et al.62 reported that there were no differences in the prognosis of the renal allograft among those who developed ABU after transplant and those who did not, though treatment of ABU may have had an impact on reducing the incidence of pyelonephritis in post-renal transplant recepients.63
The challenge lies in dealing with patients admitted to intensive care units who tend to have ABU due to prolonged catheterisation. In such patients, it is difficult to differentiate between colonisation or true infection. Also, in neutropenics and transplant individuals, the relevance of ABU is still unclear, as this could be either a colonisation or a focus of invasive infection, though this could possibly be a risk factor for patients with ABU to develop symptomatic UTI.50 In the absence of considerable evidence that antibacterial therapy of ABU can reduce infectious complications in such cases, treating with antibiotics is still considered a viable option for many clinicians. More prospective studies are needed to shed light on these aspects.
Several studies to date have clearly shown that antimicrobial treatment of ABU does not reduce the frequency of ABU. Prospective cohort studies, as well as randomised controlled studies, including patients with spinal cord injury, patients with diabetes, non-pregnant women, elderly people, and individuals with chronic catheters, have failed to show any benefits of treatment of ABU. In fact, such unwarranted treatment has resulted in subsequent isolation of resistant bacteria,10,17,64 collateral damage in terms of alteration of the normal gut flora resulting in increased risk of Clostridium difficile infection, and even increasing the risk of UTI by destroying the harmless colonised flora in the genitourinary tract.10,50,65 Notable observations made from many prospective randomised controlled trials indicate that eradication of these less virulent strains have led to pyelonephritis and in some cases recurrence of UTI.49,65-67 The hypothesis that such colonised, less virulent, strains offer some kind of protection by preventing virulent strains from causing infection has paved the way for the concept of ‘bacterial interference’.49,68 It needs to be emphasised here that untreated ABU is not harmful and rarely causes renal failure. Any episode of bacteriuria does not necessarily confirm a true UTI and, hence, should not be treated unless warranted. Any antibiotic exposure will eventually contribute to the phenomenon of antibiotic resistance. Hence, the use of antibiotics should be minimised through regular dialogue between the laboratory and the clinicians concerned, with emphasis on the idea of choosing wisely.69
In conclusion, for any bacteriuria to remain as ABU or a symptomatic UTI is determined by a complex interplay of organism, host, and environmental factors. Certain conditions with ABU continue to be a dilemma for most healthcare professionals. Nevertheless, caution needs to be exerted while treating most of them wherever clear-cut guidelines are not available. Unwarranted treatment of ABU with antibacterials contributes to antibiotic resistance.18 Therefore, it is important to raise the levels of awareness regarding ABU among healthcare professionals and, even more, to implement restrictions over the use of antibiotics for treatment of ABU.








