Abstract
Renal stones are a common condition causing significant morbidity and economic burden. The prevalence of urinary tract stones in the developed nations ranges from 4–20%. Renal stones are of different types, the most common being the calcium oxalate stones. Various dietary, non-dietary, and urinary risk factors contribute to their formation. Their frequent association with systemic diseases (like hypertension, diabetes, and obesity) highlights the role of dietary and lifestyle changes in their occurrence, recurrence, and possible prevention. Non-contrast computed tomography (CT) identifies almost every stone and is the preferred investigation for identification. Ultrasound has its advantages, as it is low cost and requires no radiation, but is observer dependent. Metabolic profiles (including blood calcium, phosphate, magnesium, creatinine, uric acid, sodium, and potassium) should be measured and a detailed urinalysis should be done. This review further discusses the formation in depth, and covers risk factors and management of renal stones, and lays down the importance of preventive measures to avoid their recurrence.
INTRODUCTION
Renal stones, or nephrolithiasis, are a common problem worldwide. With its increasing prevalence, they are imposing a significant economic burden for both developing and developed nations. It has been observed that renal stones are associated with systemic diseases like Type 2 diabetes mellitus, obesity, dyslipidaemia, and hypertension. Lifestyle and environmental factors contribute significantly in their formation. Presentation of renal colic is common and therefore treatment is not delayed. However, in the absence of any preventive measures >50% of renal stones may reoccur. This review summarises the pathophysiology of renal stones and discusses the clinical management for prevention and treatment of renal stones.
EPIDEMIOLOGY
Renal stones can occur at any age; the peak incidence is reported in persons aged 20–49 years. Males are affected more than females. The prevalence of urinary tract stones in the industrialised world ranges from 4–20%.1,2 In Italy prevalence of urinary tract stones was found to be 1.2%, in Scotland 3.5%, and in Spain 10.0%.3,4 They are rare in Greenland and coastal areas of Japan. In USA, the prevalence of nephrolithiasis was 10.6% in men and 7.1% in women. On average, 1 in 11 Americans develop kidney stones at least once in their lifetime.1,5 In developing countries, bladder calculi are more common than upper urinary tract calculi; the opposite is true in developed countries. It is estimated that the incidence of renal stones may increase from 40% to 56% by 2050 as a result of the effects of global warming.5
Renal stones are common in obese and diabetic individuals. The recurrence rate of renal stones is high, with 50% recurring within 5 years of the initial stone event. The factors that determine the accelerating pace of stone formation in recurrent stone formers are not well known. Therefore, in any single stone former, one cannot predict which patient will relapse, however, the natural history of stone disease and the high rate of recurrence requires careful diagnostic evaluation and early treatment.
CLASSIFICATION OF RENAL STONES
There are different kinds of renal stones and their correct identification is important in the selection of optimal treatment. The frequency of different stone type occurrence is shown in Table 1.
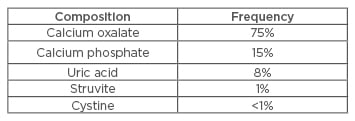
Table 1: Different types of renal stones.
There are various risk factors for the development of stones in the urinary tract, for example dietary, non-dietary, and urinary. These risk factors vary by stone type and by clinical characteristics.7,8 Major factors causing an increased risk for the development of renal stones are mentioned in Table 2.
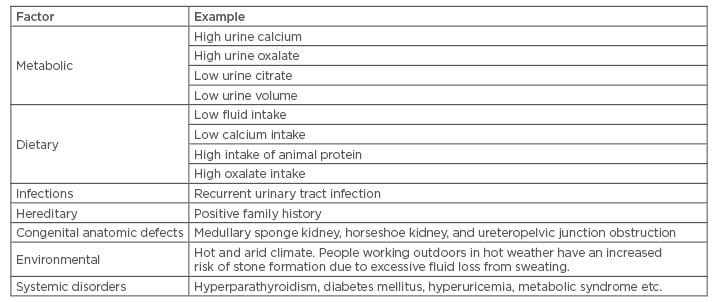
Table 2: Factors causing increased risk of renal stones.
PATHOPHYSIOLOGY
Renal stones are composed of insoluble salts from the urine and are formed by two basic mechanisms. The first mechanism is the aggregation of crystals with a non-crystalline protein (matrix) component. The salts in the urine precipitate and crystallise, aggregating the crystals, and causing them to grow into a mass sufficient to cause clinical symptoms.6 In the second mechanism, which is mostly responsible for calcium oxalate stones, deposition of stone material occurs on a renal papillary calcium phosphate nidus, typically a Randall’s plaque (which always consists of calcium phosphate).7 The majority of stones are composed of mostly calcium salts, including those of calcium oxalate and calcium phosphate. Uric acid, cystine, and magnesium ammonium phosphate (struvite) compose the remainder of the stones.
CLINICAL MANIFESTATIONS
The three narrowest parts of the ureter are at the pelvo-ureteric junction, the mid-ureter, where the ureter crosses the iliac vessels, and the vesico-ureteric junction (VUJ). The VUJ is the most common site of obstruction. Patients may present with renal colic, experiencing a severe sharp pain at the flanks which has a sudden onset, with fluctuation and intensification over 15–45 minutes. It then becomes steady and unbearable, often accompanied by nausea and emesis. As the stone passes down the ureter towards the bladder, flank pain changes in a downward direction towards the groin. When the stone is lodged at the VUJ, urinary frequency and dysuria may appear. The pain may clear as the stone moves into the bladder or from the calyceal system into the ureter. Stones may obstruct the urinary tract and impair renal function. There is an increased risk of infection with chronic obstruction. Bleeding may be chronic and accompany obstruction. The presence of bleeding alone does not predict a more severe outcome. Episodes of rapid onsets of pain, bleeding, and then rapid clearing, often known as ‘passing gravel’, are the result of passing a large amount of crystals of calcium oxalate, uric acid, or cystine. Some patients experience painless haematuria.9,10 The close differential diagnoses, which should be excluded before diagnosing renal colic, are abdominal aortic aneurysm, appendicitis, bowel obstruction, cholecystitis, mesenteric ischaemia, musculoskeletal pain, ovarian abscess, ruptured ovarian cyst, pelvic inflammatory disease, and pyelonephritis.11,12
EVALUATION OF STONE FORMERS
A detailed history and examination is required to evaluate renal stones. History of gout and recurrent urinary tract infection (UTI) should specifically be asked as hyperuricaemia in patients with gout can precipitate uric acid stones, whereas UTI can predispose the patient to struvite stones. Fasting blood calcium, phosphate, magnesium, creatinine, uric acid, sodium, and potassium should be measured.12 A detailed urinalysis should be conducted, which includes measurement of pH, albumin, glucose, 24-hour urine calcium, phosphate, magnesium, creatinine, oxalate, uric acid, citrate, and cystine.13
Imaging
All patients suspected of harbouring a stone in the urinary tract should undergo an imaging procedure to determine whether the new stone is located within the kidney parenchyma, renal pelvis, upper or lower ureter, or bladder, and whether there is ureteral obstruction.14 The localisation of stones is also important in choosing medications, surgery, or lithotripsy. Ultrasonography (USG) is used frequently to determine the presence of a renal stone. Uric acid or cystine stones are easily identifiable on USG but are not visible on plain radiograph. An advantage of USG is that it is easily available, does not require any intravenous (i.v.) contrast, and can detect hydronephrosis easily. The success of USG is dependent on the operator’s skill and experience.15 Ultrasound may not accurately visualise all stones and therefore cannot be used for follow-up to determine the appearance of new stones. Non-contrast computed tomography (CT) of the abdomen with 5 mm cuts is the most sensitive imaging technique for determining the number and location of stones within the renal parenchyma or along the upper or lower urinary tract. Using this technique, stones can be distinguished from kidney tissue or blood clots. Nephrocalcinosis can be identified as a myriad of tiny, almost microscopic, specks of radiodense calcium arrayed along the calyces. Small, separate, radiodense stones of <1 cm in diameter suggest calcium, or less commonly, cystine stones. Radiodense stones suggest either calcium or struvite composition, but struvite stones are usually large and fill the calyceal system. Cystine stones appear to be radiodense, but less dense than calcium containing stones. Small, radiolucent stones suggest uric acid composition. Uric acid stones appear as filling defects on i.v. pyelography. Filling defects that occupy the renal pelvis are staghorn stones and may be of struvite, uric acid, or cystine composition. Sludge may be of either uric acid or cystine, which can fill the renal pelvis and cause obstruction. Plain radiographs of the abdomen can identify large radiopaque stones of ≥3 mm.16
MANAGEMENT
Management of Acute Colic
Treatment of renal colic in the emergency setup involves i.v. fluids, analgesics and anti-emetic medication, and anti-emetic medication. When the diagnosis of renal colic is established, presence of obstruction or infection should be determined. In 2016, the American Urological Association (AUA) and the Endourological Society issued general management guidelines for the various presentations of stones that can be managed conservatively. The guidelines state that observation, with or without medical expulsive therapy, should be offered to patients with uncomplicated distal ureteral stones that are ≤10 mm in diameter. The guidelines also state that active surveillance can be offered for asymptomatic, non-obstructing calyceal stones. Urological intervention should be sought if there is evidence of UTI, a stone >8 mm in diameter, any anatomic abnormality, or intractable pain.17,18
Prevention of New Stone Formation
Without medical treatment, the 5-year recurrence rate is high, ranging from 35–50% after an initial stone event. A high fluid intake, enough to produce at least 2.5 L of urine per day, should be the initial therapy to prevent stone recurrence.19 Recommendations for preventing stone formation depend on the stone type and the results of metabolic evaluation. After remediable secondary causes of stone formation (e.g. primary hyperparathyroidism) are excluded, the focus should turn to modification of the urine composition to reduce the risk of new stone formation. Dietary modifications have a major role in the management of recurrent stones that are due to hypercalciuria. Dietary calcium should not be restricted, since calcium reduces the excretion of urinary oxalate by decreasing intestinal absorption of oxalate. Guidelines from the AUA recommend a daily calcium intake of 1,000–1,200 mg. Moreover, restriction of dietary calcium to <800 mg/day (the current recommended daily allowance for adults) can lead to negative calcium balance and bone loss. Sodium intake also influences hypercalciuria. Calcium is reabsorbed passively in the proximal tubule due to the concentration gradient created by active reabsorption of sodium. A high sodium intake causes volume expansion, leading to a decrease in proximal sodium and calcium reabsorption and enhancing calcium excretion. A low-sodium diet (80–100 mmoL/day, or 1,800–2,300 mg/day) is recommended.20 This enhances proximal sodium and passive calcium absorption and leads to a decrease in calcium excretion. Dietary protein increases the acid load by production of sulphuric acid and leads to hypercalciuria by its action on bone and kidney. Animal protein has a higher content of sulphur and generates a higher acid load compared to vegetable protein, with animal protein associated with an increased incidence of stone formation. It has been seen that the combination of restricted intake of animal protein (52 g/day), restricted salt intake (50 mmoL, or 2,900 mg/day of sodium chloride), and normal calcium intake (30 mmoL/day, or 1,200 mg/day) was associated with a lower incidence of stone recurrence in men with hypercalciuria, compared with traditional low-calcium intake (10 mmoL, or 400 mg/day). Patients should therefore be advised to avoid excessive intake of animal protein.21
Specific Recommendations for Different Types of Stones22-24
Calcium oxalate stones
A reduction in urine oxalate reduces the supersaturation of calcium oxalate. In patients with the common form of nephrolithiasis, avoiding high-dose vitamin C supplements is the only known strategy that reduces endogenous oxalate production. Firstly, foods that contain high amounts of oxalate should be avoided e.g. spinach, rhubarb, and potatoes. The absorption of oxalate is reduced by higher calcium intake; therefore, individuals with higher than desired urinary oxalate should be counselled to consume adequate calcium. Citrate is a natural inhibitor of calcium oxalate and calcium phosphate stones. More consumption of foods that are rich in alkali (i.e. fruits and vegetables) should be encouraged.
Calcium phosphate stones
Calcium phosphate stones share the same risk factors as with calcium oxalate stones like higher concentrations of urine calcium and lower concentrations of urine citrate. There are no current randomised trials to base preventive recommendations for calcium phosphate stone formers, so the interventions are focussed on modification of the recognised risk factors. Reduction of dietary phosphate may be beneficial by reducing urine phosphate excretion.
Uric acid stones
The mainstay of prevention of uric acid stone formation entails increasing urine pH. While acidifying the urine can be challenging, alkalinising the urine can be readily achieved by increasing the intake of foods rich in alkali (e.g. fruits and vegetables) and reducing the intake of foods that produce acid (e.g. animal flesh). Supplementation with bicarbonate or citrate salts (preferably potassium citrate) can be used to reach the recommended pH goal of 6–7 throughout the day and night.
Struvite stones
These stones require complete removal by a urologist. New stone formation can be avoided by the prevention of UTIs.
Pharmacological Intervention
- For calcium oxalate and calcium phosphate stones thiazide diuretics (with sodium restriction) may be used to reduce urine calcium25
- In patients with low urine citrate levels, alkali supplements (e.g. potassium citrate) may be used to increase these concentrations. However, the urine pH of these patients should be monitored carefully because supplemental alkali can raise urine pH, thereby potentially increasing the risk of stone formation
- Calcium channel blockers along with prednisolone have been found to facilitate ureteral stone passage and can be used in patients harbouring stones in the ureter26,27
- Tamsulosin, an alpha-1 selective blocker, is usually indicated for the treatment of lower urinary tract symptoms due to prostatic enlargement. It has also shown positive results in facilitating passage of ureteral stones27-29
- Long-term dietary cystine restriction is not feasible and is unlikely to be successful; hence for the prevention of cystine stones, treatment is done with medication that covalently binds to cystine (tiopronin and penicillamine) and a medication that raises urine pH30
Surgical treatment
Usually, stones ≤4 mm in diameter pass spontaneously and stones >8 mm are unlikely to pass without surgical intervention. Primary indications for surgical intervention according to AUA guidelines are given in Table 3.

Table 3: Indications for surgical intervention.
According to the 2005 AUA and 2016 AUA/Endourological Society guidelines:18
- Ureteroscopy (URS) is considered the first-line therapy for mid distal ureteral stone
- Percutaneous nephrolithotomy (PCNL) as the cornerstone of management for staghorn calculi
- Extracorporeal shockwave lithotripsy (ESWL)/ URS for non-lower pole stones with a total stone burden <20 mm or lower pole renal stone <10 mm
PCNL was developed to reduce the morbidity and mortality associated with open renal surgery, and it currently remains the first-line treatment for large renal stones. However, it represents the most morbid of the minimally invasive endoscopic surgeries for renal stones. Amongst the minimally invasive techniques the main options are ESWL and URS.31 ESWL is usually an outpatient procedure performed with analgesia or sedation. A shockwave is generated and focussed on the stone. Both procedures have high success rates for all ureteric stones. The use of ESWL has increased but still PCNL has many advantages over ESWL and in some cases, URS.32 Recent advancement in minimally invasive surgery involves the novel dual wave handheld lithotripter, which is useful for bladder calculi. Stonebreaker pneumatic lithotripter is more effective for staghorn calculi.33
CONCLUSION
The increasing incidence of renal stones is adding to the morbidity and huge economic losses worldwide of this pathology. The technological advances have helped with early diagnosis and treatment. However frequent association of renal stones with metabolic diseases like hypertension, diabetes, and obesity emphasise the importance of dietary practices in their occurrence and reoccurrence. High fluid intake and adopting healthy lifestyle measures are some of the cost-effective measures of preventing renal stones.








