Abstract
Myopericytoma is a rare tumour arising from myopericytes. Myopericytes are transitional cells between pericytes, which are perivascular cells adjacent to capillaries, and vascular smooth muscle cells. We report a case of cutaneous myopericytoma metastasising to the right kidney. It represents one of the few cases of malignant behaviour in myopericytoma, and is the first report of a myopericytoma metastasising to the urinary tract. This case suggests that the traditional view that urinary myopericytoma are benign lesions needs to be updated.
INTRODUCTION
Myopericytoma is a rare tumour that arises from myopericytes. Myopericytes are transitional cells between pericytes, which are perivascular cells adjacent to capillaries and vascular smooth muscle cells. The term myopericytes was first used by Dictor et al.1 in 1992 to describe a subset of myofibroblasts, and a few years later, the term myopericytoma was proposed by Requena et al.2 The immunohistochemical characteristics were described by Granter et al.3 in 1998.2 Histologically, these tumours are characterised by concentric perivascular proliferation of spindle cells. Myopericytomas form a morphological spectrum of tumours with infantile haemangiomyopericytoma, solitary myofibroma, myofibromatosis, and myopericytoma; all of which show differentiation towards perivascular myoid cells or pericytes. In 2002, the World Health Organization (WHO) categorised these tumours under the group ‘pericytic/perivascular tumours’.4
We report a case of cutaneous myopericytoma metastasising to the right kidney; this represents one of the few cases of a malignant behaviour in a myopericytoma, and the first case of a myopericytoma further metastasising to the urinary tract.
CASE REPORT
In November 2012, a 47-year-old woman consulted her dermatologist about a skin lesion in the upper middle quadrant of the right breast. The lesion was only slightly elevated and not well demarcated, with a purple angiomatous aspect. As the skin lesion was rapidly growing and presented an aesthetic problem, the woman was referred to a plastic surgeon for excision.
The lesion and a part of the underlying subcutis were removed with a minimal, lesion-free resection margin in December 2012. Microscopic examination showed a myopericytoma with atypical morphological features. Due to the presence of a high mitotic index (14/10 high power fields) and the presence of satellite nodules in the subcutaneous tissue, as well as uncertainty of the biological behaviour of the lesion, a broad resection was performed. The section margins were tumour free and no additional staging was performed.
In 2014, the patient consulted a second plastic surgeon for aesthetic correction of the postoperative scar and palpation of a new mass underneath this scar. Clinical examination showed a hard, well-circumscribed mass in the right upper quadrant of the right breast. Clinically, the tumour was not adherent to the skin but possibly adherent to the underlying pectoralis muscle. A very broad resection of the lesion was performed with stripping of a pectoralis muscle patch. Anatomopathological results revealed necrotic material, which was impossible to differentiate, surrounded by an inflammatory reaction. Resection margins were free of tumour and no additional staging was performed.
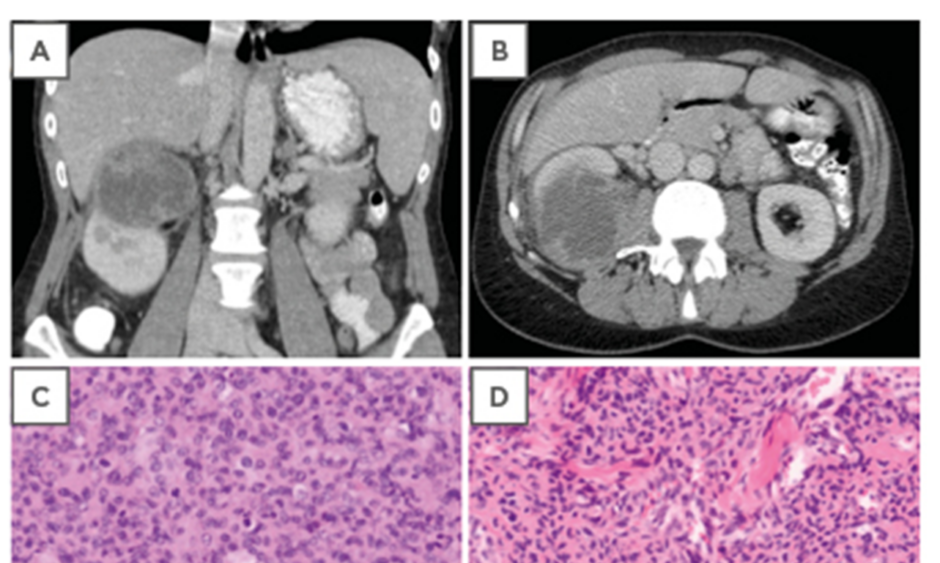
In July 2015, the patient was referred to the department of internal medicine by her family doctor with weight loss of 8 kg, persistent fatigue, and pain in the right hypochondriac region. There were no urinary symptoms. Clinical examination revealed tenderness in the right hypochondriac region, and ultrasonography showed a mass on the upper pole of the right kidney. A computed tomography (CT) scan of the abdomen revealed a voluminous tumour of 8.2 cm in the upper pole of the right kidney. The tumour showed irregular contrast captation and was assessed as a malignant solid renal tumour, presumably renal cell carcinoma. No pathologically enlarged retroperitoneal nodes were seen (Figure 1A and 1B). A CT image of the thorax showed no abnormalities. A radical right nephrectomy was performed with an uncomplicated postoperative course.
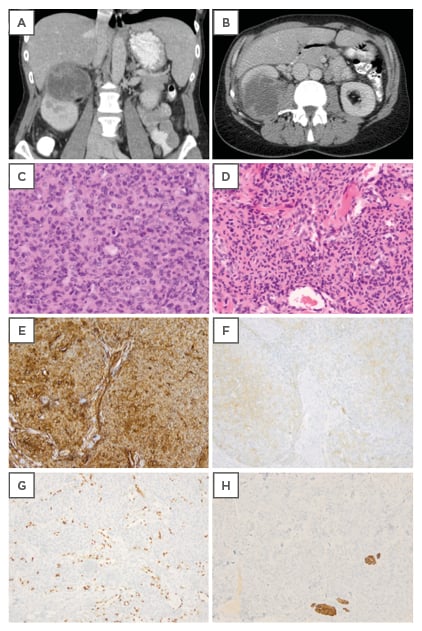
Figure 1: A,B) Computed tomography scan showing a malignant solid renal tumour of the kidney with irregular contrast captation. C) Haematoxylin and eosin staining of the kidney lesion showing a highly cellular tumour (100x). D) Hematoxylin and eosin staining of the original skin lesion showing morphology comparable to the kidney lesion. E) Immunohistochemical expression of CD34 in the original skin lesion. F) Focal immunohistochemical expression of alpha smooth muscle actin in the original skin lesion. G) Negative desmin control with internal positive control of arrector pili muscles in the original skin lesion. H) Negative ETS-related gene control of the original skin lesion with colouring of the tumour vasculature.
Pathologic examination, however, showed no renal cell carcinoma, but a high-grade sarcomatous lesion. The tumour cells were polygonal or spindle shaped, with areas of necrosis and numerous mitoses. The cells were arranged in short fascicles, sometimes with a hemangiopericytoma-like pattern (Figure 1C). The kidney lesion was compared with the original skin tumour and showed similar morphology upon haematoxylin and eosin staining (Figure 1D), and immunohistochemistry (Figure 1E–H).
Immunohistochemical analysis revealed expression of CD34 (Dako Clone QBEnd 10) (Figure 1E), and only focal expression of myogenic markers such as smooth muscle actin (Dako Clone 1A4) (Figure 1F) and calponin (Dako Clone CALP). The tumour was negative for desmin (Dako Clone D33) (Figure 1G) and the ETS-related gene (Dako Clone EP111) (Figure 1H). These findings are compatible with a high-grade sarcoma with myopericytic differentiation. Both tumours showed a high mitotic index. The initial skin tumour showed low-grade cytonuclear atypia, while the kidney tumour showed a high grade of atypia.
Next generation sequencing was performed on the kidney lesion but no BRAF mutation was present.5 Resection margins were free of tumour and no adjuvant therapy was given. A positron emission tomography (PET) CT conducted after 3 months (October 2015) showed no evidence of disseminated disease so far.
DISCUSSION
A myopericytoma is a rare tumour mostly arising in skin or superficial soft tissues. The largest case series of 54 patients was described by Mentzel et al.5 in 2006. In their series, 26 cases occurred in the lower extremities, 16 in the upper extremities, 4 in the head and neck region, and 2 in the trunk.5 The presence of myopericytoma in visceral organs is rare, although occasional cases have been described in the thorax, lungs, heart, gastrointestinal tract, brain, and urinary tract.6 Myopericytomas may have a tendency to recur locally, but most are benign tumours with an excellent prognosis.
Our case report of a myopericytoma is exceptional because of its location, namely the urinary tract. A search of English literature using the Medline database via PUBMED revealed 11 other cases of urinary myopericytoma (Table 1).7-11 Within this literature, all but one of these tumours occurred in the kidney; in one case the urinary bladder was affected. The majority of the tumours were small, with a mean diameter of 5.5 cm and a median diameter of 4.5 cm. Commonly, the lesions were incidental findings on imaging studies; though, in one case of a large mass of the left kidney, the lesion was found during clinical examination. Frequency, dysuria, or abdominal/flank pain have also been described. The age of patients diagnosed with urinary myopericytoma was variable, with the youngest patient being a 33-year-old male. None of these patients developed any evidence of recurrent or metastatic disease after surgical removal of the lesion (mean follow-up: 30 months).
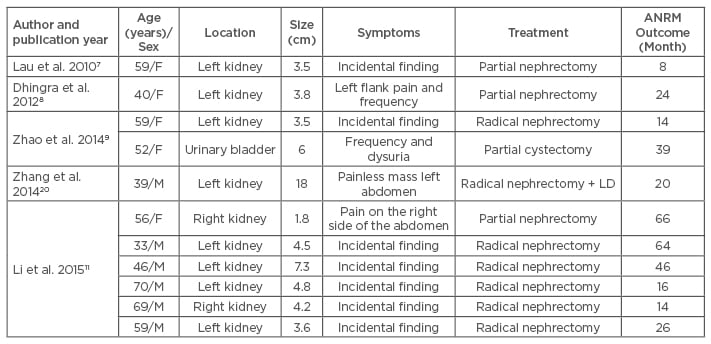
Table 1: Myopericytoma of the urinary tract.
F: female; M: male; LD: lymph node dissection; ANRM: alive with no evidence of recurrent or
metastatic disease.
Our case concerned a malignant myopericytoma. In contrast to the typical myopericytoma, this tumour has atypical features such as a high mitotic index, nuclear pleomorphism, necrosis, and high cellularity. In 2002, McMenamin and Fletcher12 named these tumours malignant myopericytomas. Incidence of these tumours is extremely rare; to our knowledge, only eight cases have been described in the literature (Table 2).5,12-14 The majority of these tumours appear to be located superficially, with only the skin and subcutaneous tissue affected. To date, only single cases of malignant mediastinal myopericytoma, malignant left atrial myopericytoma, and malignant intradural myopericytoma have been described.12-14
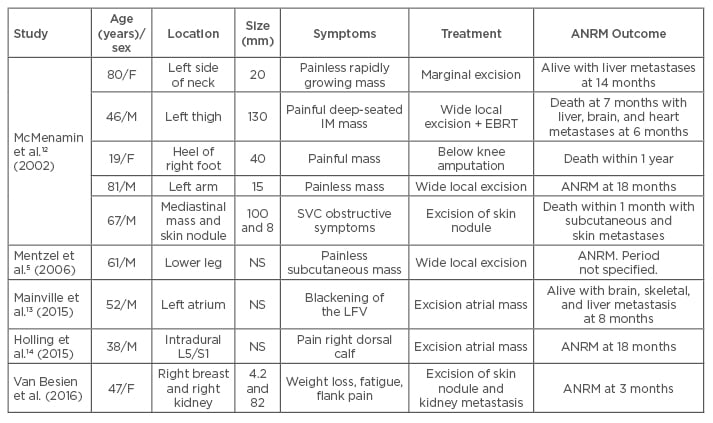
Table 2: Cases of malignant myopericytoma in the literature.
F: female; M: male; L5: lumbar 5; S1: sacral 1; NS: not specified; IM: intermuscular; SVC: superior vena cava; LFV: left field of vision; EBRT: external beam radiation therapy; ANRM: alive with no evidence of recurrent or metastatic disease.
Although metastasis of a malignant myopericytoma to the liver, brain, skeletal system, and heart have previously been described, this report represents the first described case of a metastasis of a malignant myopericytoma in the urinary tract.
Benign myopericytomas are diagnosed by excisional biopsy. The pathological result is often a surprise to the treating physician due to the rarity of these tumours. Diagnosis is further confirmed by immunohistochemical analysis as myopericytes are immunoreactive for smooth muscle actin and CD34, but rarely for desmin.6 However, no data exist for the follow-up or staging of these tumours. In cases of superficial myopericytoma with classic histological features, complete excision is curative. When these tumours are located in visceral organs additional staging is often performed by imaging studies. However, the prognosis of benign myopericytomas tends to be good.
Malignant myopericytomas may present as a primary metastatic disease, or may rapidly metastasise as described above. Broad excision of these lesions with high mitotic index is the treatment of choice. Despite broad resection, the prognosis of a malignant myopericytoma remains unclear. Out of the five cases described by McMenamin and Fletcher,12 three patients died within the first year. The two surviving patients showed varying status; one developed liver metastases during the follow-up period of 14 months, and the other survived with no evidence of recurrent or metastatic disease after surgical removal of the lesion. In another report from 2006, a subcutaneous malignant myopericytoma of the lower leg was treated by resection without any evidence of recurrent or metastatic disease. The follow-up period was undefined.5 Two recent papers have reported cases of malignant myopericytomas, one in the left atrium and one in the dura mater. Of these papers, the former reported metastases to the brain, skeletal system, and liver after 8 months, while the latter paper reported no evidence of recurrent or metastatic disease after 18 months.13,14
Until recently, there were no data available about the value of adjuvant therapy in preventing local recurrence or metastases of this disease. One case report described wide excision of a deep-seated, intermuscular malignant myopericytoma followed by external beam radiation. However, the patient died after 7 months due to metastases to the liver, brain, heart, and skeletal system.12 Recent genetic testing has, in some cases, revealed BRAFV600E mutations.15 These mutations are present in 15% of benign myopericytomas, 33% of which were multifocal/infiltrative or recurrent. Anti–BRAFV600E therapy with vemurafenib disrupts angiogenetic and metabolic properties with downreglation of some extracellular matrix factors. Consequently, these molecules might be a useful adjuvant treatment for myopericytomas expressing BRAFV600E mutations. Screening for these mutations is therefore recommended.
In our case report, the patient presented with a myopericytoma of the skin with atypical features. A broad resection was performed but no additional imaging studies were performed. In the years following, a tumour in the right kidney with similar histological features as the skin lesion but a higher degree of cytonuclear atypia occurred. We decided to perform a resection of a kidney metastasis of a malignant myopericytoma of the skin. It is possible that the kidney lesion was already present when excision of the skin lesion was performed and gradually grew until it became symptomatic. As the kidney lesion had higher cellularity, an elevated mitotic index and more cytonuclear atypia, as well as necrosis, we believe that the kidney tumour was a metastasis and not the primary tumour. As no BRAFV600E mutation was found, and no other site of metastatic deposit was seen, no adjuvant therapy could be given. Three months after excision of the kidney metastasis a PET CT scan showed no evidence of metastases.
CONCLUSION
We report the first case of a malignant myopericytoma with metastases to the kidney. Therefore, the traditional view that urinary myopericytoma are benign primary lesions needs to be updated.