This symposium took place on the 1st July 2022 as part of the 37th Annual European Association of Urology (EAU) Congress.
Chairperson: Axel Bex1
Speakers: Tom Powles,2 Viktor Grünwald,3 Michael Staehler4
1. The Royal Free London NHS Foundation Trust, UK
2. Barts Cancer Institute, Queen Mary University, London, UK
3. University Hospital Essen, Germany
4. University Hospital, Ludwig Maximilian University of Munich, Germany
Disclosure: Bex has received honoraria, consultancy/speaker fees, and/or educational grants from BMS, Eisai, Pfizer, and Roche-Genentech. Powles has received honoraria, consultancy fees, and/or educational grants from Astellas, AstraZeneca, BMS, Eisai, Exelixis, Incyte, Ipsen, Johnson & Johnson, Merck, Merck Serono, MSD, Novartis, Pfizer, Roche, and Seattle Genetics. Grünwald has received honoraria/consultation fees, grants/research support, participated in speaker bureaus, is a stock shareholder, and/or has received travel support from Apogepha, AstraZeneca, BMS, Debiopharm, Eisai, EUSAPharm, Genmab, Ipsen, Janssen, Merck Serono, MSD, Nanobiotix, Novartis, ONO Pharmaceutical, Pfizer, Roche, and Seagen. Staehler has received honoraria, consultancy/speaker fees, and/or research funding from Apogepha, Astellas, Aveo, Bayer, BMS, EISAI, EUSAPharm, Exelixis, GlaxoSmithKline, Immatics, Ipsen, MSD, Novartis, Pelloton, Pfizer, Roche/Genentech, and Wilex.
Acknowledgements: Writing assistance was provided by Helen Boreham, Wetherby, UK. Product characteristics for Eisai products mentioned in this article: Kisplyx (lenvatinib) is indicated for the treatment of adults with advanced renal cell carcinoma in combination with pembrolizumab as first-line treatment, or in combination with everolimus, following one prior vascular endothelial growth factor-targeted therapy. Kisplyx prescribing information available here
Support: The publication of this article was initiated and fully funded by Eisai Europe Ltd. This is a promotional material, and the content is intended for healthcare professionals only, and the company products are discussed. MSD has no involvement in this initiative other than reviewing the scientific content for accuracy and compliance with regulations. Prescribing information is available at the end.
Disclaimer: This article is promotional material intended for UK healthcare professionals.
Citation: EMJ Urol. 2022;10[Suppl 2]:2-10. DOI/10.33590/emjurol/10050602. https://doi.org/10.33590/emjurol/10050602.
Meeting summary
As part of the 37th Annual European Association of Urology (EAU) Congress, this symposium presented key data from the CLEAR study: a Phase III randomised controlled trial evaluating lenvatinib plus pembrolizumab versus sunitinib in patients with advanced renal cell carcinoma (RCC) in the first-line (1L) setting.1 Leading experts discussed strategies for optimising treatment outcomes in RCC using lenvatinib plus pembrolizumab and considered how to identify the right patients for this combination therapy in clinical practice.
Advanced Renal Cell Carcinoma: How Are We Doing?
Axel Bex
Axel Bex from The Royal Free London NHS Foundation Trust, UK, gave an update on the current treatment landscape in advanced RCC. Despite the introduction of immuno-oncology (IO) combination therapies around half a decade ago, the majority of patients with Stage IV metastatic RCC still do not receive a second-line (2L) systemic therapy. This was shown in a 2020 survey of 103 physicians from across five European countries treating over 4,500 patients monthly, of which 53.7% did not receive 2L systemic treatment (data on file, presented at EAU 2022). Of these patients, 17.9% progressed but did not receive 2L treatment, 16.3% died before receiving further treatment, and 19.5% were in long-term response (data on file, presented at EAU 2022). These findings highlight the need to further advance the field and progress our knowledge, noted Bex.
According to recently updated 2022 EAU RCC guidelines, IO and tyrosine kinase inhibitor (TKI) combinations are now recommended as 1L therapy for metastatic clear cell RCC (ccRCC) in both International Metastatic RCC Database Consortium (IMDC) favourable-risk and intermediate-/poor-risk patients; with previous standards of care sunitinib and pazopanib relegated to alternative treatments.2 Similar recommendations are mirrored in the updated 2021 European Society for Medical Oncology (ESMO) guidelines, where IO-TKIs, including the recently approved lenvatinib plus pembrolizumab combination, are recommended as 1L treatments for advanced ccRCC irrespective of IMDC risk grouping.3 Cabozantinib, sunitinib, and pazopanib are again alternative treatments.3 This is good for users of these guidelines, commented Bex, because the indications and the recommendations for these combination therapies are similar.
Lenvatinib Plus Pembrolizumab Combination in First-Line Treatment of Advanced Renal Cell Carcinoma: Key Data From the CLEAR Study
Tom Powles, Viktor Grünwald, and Michael Staehler
Tom Powles from Barts Cancer Institute, Queen Mary University, London, UK, began by outlining the mechanism of action of lenvatinib and the rationale for combining TKIs and immune checkpoint inhibitors in RCC. Lenvatinib is a broad-spectrum TKI targeting vascular endothelial growth factor (VEGF) receptors 1–3 but also other important proangiogenic and oncogenic pathway-related receptor tyrosine kinases, including the fibroblast growth factor (FGR) receptor family, KIT, and RET.4,5 Pembrolizumab is an immune checkpoint inhibitor targeting programmed cell death 1 (PD-1).5 Powles highlighted the additivity and synergy that has been seen between these two agents. In syngeneic mouse tumour models, lenvatinib decreased tumour-associated macrophages, increased activated cytotoxic T cells, and demonstrated greater antitumour activity in combination with an anti-PD-1 monoclonal antibody compared with either treatment alone.6,7
VEGF receptor inhibition and PD-1/programmed death-ligand 1 (PD-L1) blockade are accepted strategies for treating RCC.8 Dysregulated VEGF signalling is a pivotal driver of angiogenesis, immune suppression, and tumour progression.9 But we also know that VEGF affects the immune repertoire, stated Powles, and overexpression of VEGF can induce immunosuppression in the tumour microenvironment through both innate and adapted immune components.9 PD-L1, commonly upregulated in RCC, inhibits local antitumour T cell-mediated responses.10 Therefore, the rationale for the combination lies in the fact that, by giving TKI and immune therapy together, we can potentially enhance the immune response and the VEGF targeting, explained Powles.
Preclinical data show lenvatinib to be a “very active targeted therapy,” remarked Powles, delivering potent inhibition of both VEGF and FGF receptor in human thyroid cancer xenograft models.11 IC50 values for FGF targeting were 27‒61 nM for lenvatinib compared with 150‒3,400 nM for sorafenib. Lenvatinib, but not sorafenib, also demonstrated significant inhibition of both VEGF- and FGF-driven angiogenesis in an in vivo model.11
The CLEAR trial of lenvatinib plus pembrolizumab was a Phase III, randomised, controlled study enrolling a total of 1,069 patients with advanced RCC in the 1L setting (NCT02811861).1,6,12 Approximately 350 previously untreated patients were randomised into each of the study’s three arms: lenvatinib plus pembrolizumab, lenvatinib plus everolimus, and a sunitinib control arm. A 1L use of lenvatinib plus everolimus is not approved in patients with advanced RCC but was included for transparency as a second experimental arm. The primary endpoint of the CLEAR trial was progression-free survival (PFS), with objective response rate (ORR), overall survival (OS), and tolerability evaluated as key secondary endpoints. Powles described the characteristics of enrolled patients as ”very much in line with what you would expect for 1L clear cell RCC,” with a 33%, 56%, and 10% spread across favourable, intermediate, and poor IMDC risk categories, respectively. The median patient age was 62 years, the majority (75%) were male, and 44% were aged ≥65 years. Common sites of metastases were the lung (69%), lymph nodes (46%), and bone (24%).1,6
In the CLEAR study, the combination of lenvatinib plus pembrolizumab proved superior to sunitinib for the primary endpoint of PFS, with a 61% relative reduction in the risk of disease progression or death (hazard ratio [HR]: 0.39; 95% confidence interval [CI]: 0.32–0.49; p<0.001 (Figure 1)). In terms of absolute risk, the number of patients with an event was 45% with lenvatinib plus pembrolizumab versus 57% with sunitinib. Median PFS was 23.9 months for the lenvatinib plus pembrolizumab arm compared with 9.2 months for sunitinib, so “a significant increase in PFS associated with the lenvatinib and pembrolizumab combination,” noted Powles.1,6
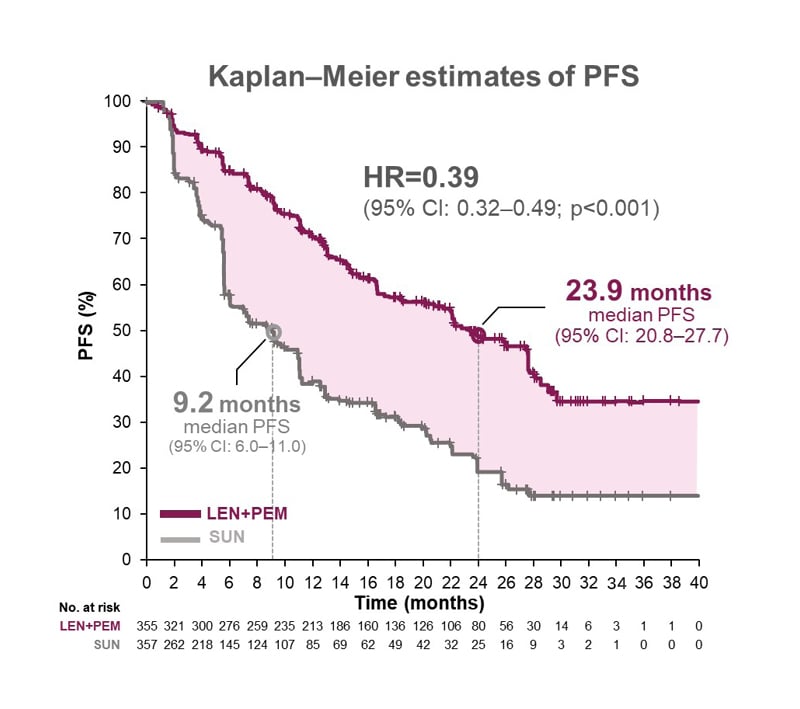
Figure 1: CLEAR trial: superior progression free survival with lenvatinib plus pembrolizumab versus sunitinib.
CI: confidence interval; HR: hazard ratio; LEN: lenvatinib; No.: number; PEM: pembrolizumab; PFS: progression-free survival; SUN: sunitinib.
Adapted from Motzer et al.1
In a prespecified subgroup analysis of PFS, lenvatinib plus pembrolizumab outperformed sunitinib across broad subgroups of patients, irrespective of geography, IMDC score, performance status, number of metastatic sites, or indeed the PD-L1 biomarker, remarked Powles.1 This outperformance was maintained in an exploratory subgroup analysis of patients with or without adverse prognostic features such as sarcomatoid, prior nephrectomy, liver metastases, and bone metastases.13 However, it should be noted that these subgroup analyses were not powered to detect differences in treatment effect.
OS at 26.6 months median follow-up was also superior with lenvatinib plus pembrolizumab versus sunitinib, with an HR of 0.66 (95% CI: 0.49–0.88; p=0.005), which is equivalent to a significant 34% relative reduction in the risk of death (Figure 2). Median OS was not reached in either group.5,11 A more mature exploratory analysis of OS data from the CLEAR trial at 34 months of follow-up showed HR favouring lenvatinib and pembrolizumab: 0.72 (95% CI: 0.55–0.93).14
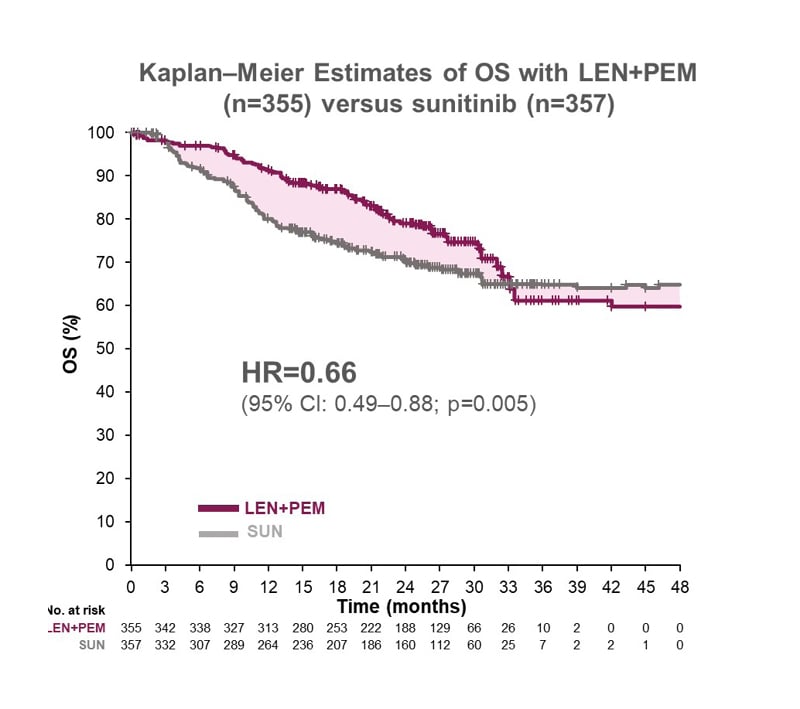
Figure 2: CLEAR trial: superior overall survival with lenvatinib plus pembrolizumab versus sunitinib
CI: confidence interval; HR: hazard ratio; LEN: lenvatinib; No.: number; OS: overall survival; PEM: pembrolizumab; SUN: sunitinib.
Adapted from Motzer et al.1
Powles went on to present 6-month landmark analysis from the CLEAR trial of OS by the depth of response, with patients stratified into quartiles according to the percentage of confirmed complete response (CR).15 Overall, 12.4% of patients in the lenvatinib plus pembrolizumab arm had confirmed CR or >75% reduction in target lesions, compared with 4.5% in the sunitinib arm. The OS rate at 2 years was similar among patients treated with lenvatinib plus pembrolizumab who had a confirmed CR or >75% reduction in target lesions.15 So you can see that patients getting CR or deep responses with lenvatinib plus pembrolizumab are doing “well” in terms of survival probability, remarked Powles.
Looking at the ORR in the CLEAR trial, responses were nearly twice as high for lenvatinib plus pembrolizumab (71.0%) versus sunitinib (36.1%), with CR rates of 16.1% and 4.2%, respectively (p<0.0001). Importantly, only 5.4% of patients on lenvatinib with pembrolizumab experienced progressive disease as best response compared with 14% on sunitinib. In addition to significantly improved ORR, the duration of response was also prolonged for lenvatinib plus pembrolizumab over sunitinib: 25.8 months and 14.6 months, respectively.1,6 Finally, Powles presented a subset analysis showing an improved ORR with lenvatinib plus pembrolizumab versus sunitinib across IMDC risk groups and the intent-to-treat population.15 From both a PFS and response perspective, you can see ”clear benefit” for the combination of lenvatinib and pembrolizumab, Powles concluded.
Panel Discussion
In the ensuing panel discussion session, experts were posed relevant questions on the CLEAR trial data and its clinical context.
What is the significance of the CLEAR efficacy data? Do they differentiate lenvatinib plus pembrolizumab from other first-line advanced clear cell renal cell carcinoma combinations?
In Michael Staehler’s, University Hospital, Ludwig Maximilians, University of Munich, Germany, opinion, the CLEAR data show a “high rate of local control, which we haven’t seen so far in the other combinations.” He added that tumour control is crucial for patients in determining the duration of their PFS. Powles agreed that the response and PFS data for lenvatinib plus pembrolizumab were “compelling” and a “good reason” to use this combination. He also highlighted the low rate of progressive disease in the lenvatinib plus pembrolizumab arm, with over 90% of patients achieving disease control with the combination.1 Progression of the disease is a “disaster” for patients, so this underlines an important efficacy signal for lenvatinib plus pembrolizumab, Powles added.
With long-term overall survival data emerging for other combination therapies in advanced renal cell carcinoma, how impactful do you find the current CLEAR study data?
CLEAR has shown the OS benefit of lenvatinib plus pembrolizumab in the intent-to-treat population, and the HR is almost equal to 0.7,1 which is similar to that reported for other combinations, commented Powles. All the experts agreed that, for patients, living as long as possible was key but also stressed that tumour control, response rates, and PFS are important surrogate outcome measures connected to OS. The panel felt the strength of these other efficacy parameters was particularly crucial in the context of treating IMDC favourable-risk patients, where trials of combinations have not shown clear OS benefits and the data remain immature.
What impact do you think the CLEAR data will have on the current approach of treating the following patient subgroups: International Metastatic Renal Cell Carcinoma Database Consortium favourable-risk, sarcomatoid renal cell carcinoma, liver metastases, and bone metastases?
Early surrogates are “pointing in the right direction” for lenvatinib plus pembrolizumab, noted Powles, and “may hit survival in the future in this subgroup.” Viktor Grünwald from the University Hospital Essen, Germany, agreed, noting that “for lenvatinib plus pembrolizumab, we have seen outstanding PFS data and a high number of complete responses, making a strong case for the combination in a good-risk patient population.” Panel consensus was that IO-TKIs, including the lenvatinib plus pembrolizumab combination, are suitable treatment options for patient subgroups with sarcomatoid RCC, as well as liver and bone metastases.
Optimising Treatment Outcomes in Advanced Renal Cell Carcinoma with Lenvatinib Plus Pembrolizumab
Tom Powles, Viktor Grünwald, and Michael Staehler
In the next session of the symposium, Powles gave an overview of key safety data from the CLEAR trial (Table 1). Median duration of therapy was longer for lenvatinib and pembrolizumab (17.0 months) than sunitinib (7.8 months), so “inevitably” there will be an accumulation of adverse events (AE), noted Powles, adding that the combination is also “more complex because it’s two drugs rather than one.” Grade ≥3 AEs occurred in 82.4% of patients receiving lenvatinib plus pembrolizumab and 71.8% on sunitinib. Discontinuations due to treatment-emergent AEs in both arms were 37.2% (either lenvatinib or pembrolizumab or both) and 14.4% (sunitinib).1 Patients with any grade treatment-emergent AEs leading to dose interruption of lenvatinib, pembrolizumab, or both drugs totalled 78.4% versus 53.8% for sunitinib, while dose reductions for lenvatinib only were 68.8% versus 50.3%.1 The take-home message from this is that education and training around giving the drugs and managing the toxicity are vitally important, stressed Powles.
Looking at the nature of AEs in the CLEAR study, Powles described them as “characteristic of previous studies of VEGF TKI and PD-1 combination therapy,” including diarrhoea, hypertension, fatigue, nausea, and stomatitis.1 The timing of these AEs was explored in a post-hoc analysis of the CLEAR safety data. It showed hypertension, dysphonia, fatigue, and stomatitis onsetting early (within the first 6 weeks of treatment on average), with other common AEs such as diarrhoea occurring later (median 20 weeks).16
Impact on quality of life (QoL) was explored in the CLEAR trial using the standard patient-reported outcome measures: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30), the EuroQoL-5-Dimension Questionnaire-3-level (EQ-5D-3L), and the Functional Assessment of Cancer Therapy‒Kidney Symptom Index‒Disease-Related Symptoms (FKSI-DRS).16 Patients receiving lenvatinib plus pembrolizumab showed similar or better health-related QoL scores than those on sunitinib. Lenvatinib plus pembrolizumab was associated with a more than 12-week delay in median time to worsening global health status, physical functioning, and patient-reported symptoms with no subsequent recovery versus sunitinib.16 These data are not conclusively showing better QoL with the combination, commented Powles, but some of the points trend towards that direction.
In addition, Powles noted that the recommended starting dose of lenvatinib is 20 mg daily, taken orally with or without food, while pembrolizumab is dosed at 200 mg every 3 weeks or 400 mg every 6 weeks via intravenous infusion.6,7 Regarding therapy management, modifications to the dose of lenvatinib can help manage adverse reactions, with interruptions, dose reductions, or discontinuation of lenvatinib as appropriate. The recommended dose reductions for lenvatinib are as follows: the first reduction to 14 mg orally once daily, the second to 10 mg once daily, and the third to 8 mg once daily.6 These dose reduction options for lenvatinib can assist clinicians in optimising the safety and tolerability profile of the combination therapy without comprising efficacy, explained Powles. No dose reductions are recommended for pembrolizumab.
Panel Discussion
What should clinicians consider during dose modification of lenvatinib, particularly during the initial phase of treatment? How might the dose modification strategy vary depending on the nature and severity of the adverse reaction?
Grünwald reiterated the importance of monitoring during treatment, as different AEs may manifest at different time periods, and being alert for AEs such as endocrinopathies and hypertension during the initial phase; however, treating these AEs is relatively straightforward, he added. Diarrhoea is more complex due to the overlap of toxicity between TKI and IO components and may require withholding of the TKI to dissect out the individual effects, Grünwald noted. Specific guidance on the management of selected lenvatinib AEs, including detailed advice on monitoring and treatment plus recommended dose modifications according to severity, are outlined in the lenvatinib summary of product characteristics.6 Grünwald also mentioned the importance of monitoring for possible proteinuria with lenvatinib.
How would you describe your own experience managing adverse reactions associated with lenvatinib plus pembrolizumab?
Grünwald said he had found lenvatinib plus pembrolizumab “manageable to prescribe” and within that spectrum of IO combinations. However, the experts acknowledged that the toxicity of combinations could prove more complex to manage than single agents like pazopanib and sunitinib. In particular, the challenge of unravelling side effects due to the IO component from those caused by the VEGF TKI. Powles emphasised the importance of intervening early if toxicity does arise during treatment with lenvatinib plus pembrolizumab and being reactive to patients’ concerns.
From CLEAR Study Evidence to Clinical Practice: Identifying the Right Patient for Lenvatinib Plus Pembrolizumab Combination Therapy
Axel Bex
The third segment of the meeting consisted of two clinical case studies (hypothetical patient case studies used for illustrative purposes), during which the panel considered how to optimise therapeutic positioning and management of patients with lenvatinib plus pembrolizumab combination therapy.
Case 1
Bex introduced the first patient case study: a 62-year-old male who presented 3 years after nephrectomy with curative intent, persistent fever, and blood in the urine. Haemoglobin was 10.2 g/dL; renal function was slightly impaired with a creatinine of 1.9 mg/dL; Karnofsky performance score (KPS) was 80 with an Eastern Cooperative Oncology Group (ECOG) of 1; and chest, abdomen, and pelvic CT scan showed metastases in bilateral lungs (25 lesions in each lung of 1–3 cm), bones, lymph nodes, and liver. Biopsy indicated a ccRCC of International Society of Urologic Pathologists (ISUP) and World Health Organization (WHO) Grade 2, and the Memorial Sloan Kettering Cancer Center (MSKCC) and IMDC risk scoring was an intermediate risk. The ultimate diagnosis was, therefore, Stage IV advanced RCC.
Grünwald explained that the pattern of recurrence, risk category, and individual “real-life” factors would all play a role in determining treatment strategy for this patient. He added that the high metastatic load and multiple metastatic sites were also a key consideration, making lenvatinib plus pembrolizumab a “very good choice” for this patient. The panel was then asked how they would monitor and manage this patient if Grade 2 diarrhoea developed after 2 weeks of treatment with lenvatinib plus pembrolizumab. Staehler responded that this was a complicated question because both drugs have the potential to cause diarrhoea. As pembrolizumab is already administered and “in the system,” he suggested a lenvatinib treatment interruption, which “usually resolves the issue,” and adding in some hydrocortisone to treat the diarrhoea.
Case 2
The second case was a 72-year-old female who presented in primary care with abdominal pain. CT scan revealed three pancreatic lesions, 12 lung lesions up to 1.5 cm, and four mediastinal lymph nodes with 1.2 cm lesions. The patient had undergone a prior partial nephrectomy, and the performance score was KPS of 90 and ECOG of 0. Biopsy showed Grade 2 ccRCC with MSKCC/IMDC favourable risk.
The panel discussed the best treatment strategy for this favourable-risk patient and weighed up the decision to start systemic therapy. Staehler commented that combining lymphatic and haematogenic metastases would steer him towards expedited systemic therapy. Powles added that the three sites of disease and heavy lung burden equated to the “sword of Damocles” hanging over the patient’s head. The big decision, he said, was, therefore, between combination TKI/IO therapy or sunitinib. Although there is no clear survival benefit as it currently stands for the combination, “many feel it’s preferential because it’s better at getting deeper responses and maybe more durable responses,” Powles noted, “but we need to continue to follow those trials up.” The panel then discussed how to manage this patient assuming a CR was achieved after 4 months of lenvatinib plus pembrolizumab combination therapy. The experts highlighted the need to gather more data and tailor treatment decisions to individual patients. However, both Staehler and Powles cautioned against discontinuing therapy prematurely.
Conclusion
Concluding the symposium, Bex summarised the key findings from the CLEAR trial, which demonstrated superior PFS, ORR, and OS with lenvatinib plus pembrolizumab compared with sunitinib (data on file, presented at EAU 2022). The combination also showed an increased duration of response versus sunitinib and similar or better health-related QoL scores.1,17 Flexible management of AEs is facilitated by the dose reduction strategy of lenvatinib, which can be optimised from 20 mg down to 14 mg, 10 mg, and 8 mg.6 Patients with advanced RCC who have not been previously treated with systemic therapy are therefore appropriate for lenvatinib and pembrolizumab combination treatment regardless of IMDC risk grouping, Bex concluded.1,6
Audience Question and Answer
The final part of the symposium consisted of a live and interactive audience question and answer session. The first question asked about interpreting the PFS2 (time from randomisation to progression on subsequent therapy or death) data for lenvatinib plus pembrolizumab presented at this year’s American Society of Clinical Oncology (ASCO) meeting.18 The data show that PFS2 is better when giving the combination of lenvatinib plus pembrolizumab first, rather than sunitinib, said Powles, which would be expected because there is a survival advantage.17 Grünwald added that PFS2 is a helpful tool to illustrate the benefit gained with therapy in 1L is sustainable.
The second question centred on experts’ real-world experience with using lenvatinib plus pembrolizumab and how this compared to trial results regarding efficacy and toxicity. Grünwald explained that access to lenvatinib plus pembrolizumab has been granted in Germany, and the combination has already been offered to patients, a decision he described as “justified based on the data we have in hand.” Powles reiterated that lenvatinib plus pembrolizumab and two other TKI/IO combinations had shown a survival advantage in advanced RCC, all with HRs around the 0.7 mark, so ”in context, you can choose between the three.” He added that colleagues he has spoken to worldwide have been “attracted” to the “high response rate and impressive PFS” with lenvatinib plus pembrolizumab.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for the MHRA Yellow Card in the GooglePlay or Apple App Store, or Republic of Ireland: www.hpra.ie. Adverse events should also be reported to Eisai Ltd on +44 (0) 208 600 1400 or [email protected].
Job bag: GB-KLR-00060
DOP: October 2022








