Abstract
Benign prostatic hyperplasia (BPH) is the non-malignant growth of the prostate, commonly experienced in ageing males, and may lead to obstructive urinary symptoms. Prostatic artery embolisation (PAE) is a rapidly emerging and effective treatment for BPH, as a minimally invasive alternative to surgical options. PAE is the process of injecting embolic microspheres into the prostatic arteries via a catheter to block blood flow to the prostate, causing tissue death and consequently reducing the size of the prostate. Adequate pre- and post-procedural evaluations with clinical examinations and questionnaires, laboratory tests, and urodynamic and imaging examinations are of key importance to achieve successful treatment outcomes. Considering the increasing use of PAE, surgeons, radiologists, and interventional radiologists should be aware of the main technical concepts of PAE and the most appropriate patients for this treatment option. Despite several national and international guidelines recommending PAE, its precise role in the management of patients with BPH, long-term outcomes and multidisciplinary team management are still forthcoming. The goal of this review is to provide multidisciplinary team members with an overview of PAE to treat BPH, considerations for selecting appropriate patients, as well as the importance of collaboration across disciplines for improved outcomes with this treatment option.
INTRODUCTION
BPH is the most common non-cancerous neoplasm of ageing males,1 increasing in incidence with age. The prevalence of BPH has been estimated at approximately 8% in males aged 30–40, increasing to 90% in those ≥80 years of age.2,3 Globally, approximately 30 million males have BPH-related symptoms.3
BPH refers to a benign anatomical enlargement of the prostate causing compression of the urethra with compromised urinary flow and bladder outlet obstruction.4 This can lead to symptoms, referred to as lower urinary tract symptoms (LUTS).5 LUTS is the preferred terminology to describe a constellation of symptoms caused by multiple pathologic conditions and may be primarily voiding, storage, or mixed.6 Males with BPH may be asymptomatic; however, symptoms are more common as males age.7
The aetiology of BPH is multifactorial involving prostatic enlargement, smooth muscle hyperplasia, bladder dysfunction, and central nervous system input.8,9 Presentation can include both storage symptoms (such as frequency, urgency, nocturia, incontinence) and voiding symptoms (such as weak stream, dribbling, dysuria, and straining).5 Diagnosis (Figure 1) hinges on a thorough medical history, a focused physical examination (including an abdominal examination for a palpable bladder, a digital rectal exam [DRE] and a neurological assessment) and laboratory testing (including urinalysis, urine culture, prostate-specific antigen [PSA] level, electrolytes and creatinine). Other tests may be considered depending on presentation of symptoms, including urine cytology, imaging, cystourethroscopy, post-void residual (PVR), and pressure-flow studies.
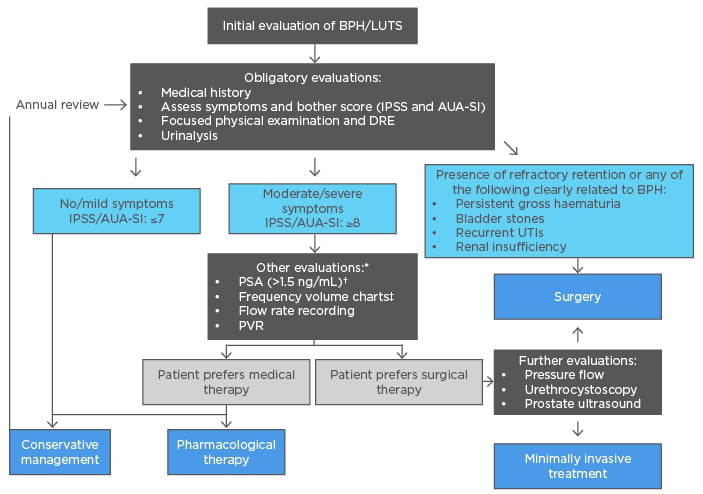
Figure 1: Diagnostic and treatment algorithm for benign prostatic hyperplasia.6,10-22
*In select patients, generally performed by urologist
†In life expectancy of >10 years, a prostate cancer diagnosis can affect the BPH management plan.
‡Used when nocturia is the primary symptom.
AUA-SI: American Urological Association Symptom Index; BPH: benign prostatic hyperplasia; DRE: digital rectal exam; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; PSA: prostate-specific antigen; PVR: post-void residual; UTI: urinary tract infection.
Furthermore, consideration of other urological conditions which may be causing LUTS, such as a tight bladder neck, an overactive bladder or a urethral stricture, is critical. The severity of BPH can be determined with the International Prostate Symptom Score (IPSS) and disease-specific quality of life (QoL) questions. The IPSS is a patient self-administered questionnaire, modified from the American Urological Association Symptom Index (AUA-SI), containing seven questions related to urinary symptoms and one question related to the patient’s perceived quality of life.6
MANAGEMENT OPTIONS FOR BENIGN PROSTATIC HYPERPLASIA
Current management strategies involve conservative management, pharmacotherapy, phytotherapy, minimally invasive treatments, and surgical interventions as indicated (Figure 1). The goal of treatment is to relieve LUTS and slow the clinical progression of BPH while improving patient QoL. Factors influencing the treatment choice for an individual patient would include patient evaluation; predicted treatment outcomes achievable by available treatment options; patient preferences; treatment expectations from the chosen treatment option in terms of speed of onset, efficacy, side effects, and QoL. A conservative management strategy could be appropriate in patients with clinical BPH, including those with mild symptoms (IPSS/AUA-SI score: ≤7); those with moderate-to-severe non-bothersome symptoms (IPSS/AUA-SI score: ≥8) and are not experiencing complications of BPH; and patients in whom medical therapy is not improving their symptoms and/or QoL.10,11 Conservative management includes lifestyle changes such as avoiding alcohol, caffeine, decongestants, and antihistamines; adapting fluid intake to daily routine; relaxation exercises and distraction techniques; losing weight; and bladder training with ongoing monitoring of symptoms.12,13 In addition, the risks of treatment may outweigh any benefits in such cases. Patients managed expectantly with a conservative management strategy are usually reviewed annually.3,11
If lifestyle modifications are insufficient in improving QoL, then pharmacotherapy may be indicated in patients who do not have absolute indications warranting an invasive procedure.14 Pharmacological therapy primarily involves drugs that either relax the smooth musculature (e.g., α1-adrenoceptor antagonists, muscarinic receptor antagonists, β3 agonists, and phosphodiesterase 5 inhibitors) or exert anti-androgen effects to reduce prostate cell proliferation (e.g., 5α-reductase inhibitors [5-ARI]).15-18 If symptoms or disease severity warrant, therapy can be initiated as mono- or combination therapy depending on symptom profile.18 α-blockers work by blocking α-1a receptors, thereby relaxing smooth muscle within the bladder neck and prostate, whilst 5-ARIs inhibit the conversion of testosterone to dihydrotestosterone, thereby precluding prostatic tissue growth and causing prostatic cell apoptosis. Patients with severe symptoms, exceptionally large prostates and/or those who failed monotherapy, may benefit from combination therapy with an alpha-blocker and 5-ARI. Phosphodiesterase 5 inhibitors are vasodilators, which increase intracellular cyclic guanosine monophosphate, causing a nitric oxide mediated reduction in smooth muscle tone of the prostate, detrusor muscle, and urethra. A low adherence to and dissatisfaction with pharmacological management regimens suggests that BPH might frequently be inadequately managed by pharmacological interventions.17 Patients should be evaluated several weeks after initiating treatment, provided adverse events do not require earlier consultation, to assess response to therapy. Re-evaluation should include the IPSS, PVR and uroflowmetry.19
Phytotherapy, plant-based or herbal medications, may be used in males experiencing mild-to-moderate LUTS. Clinical trials have shown efficacy in the treatment of LUTS; however, many products are not standardised, and long-term safety data are not always available. Options may include Serenoa repens (saw palmetto), Pygeum africanum (tree bark), Cucurbita pepo (squash), and Urtica dioica (stinging nettle).23,24 Clinicians should critically evaluate the risks and possible benefits of using alternative treatments.
Medical treatment is indicated in the first instance or in patients with mild LUTS (IPSS: <8). Invasive intervention may be required in patients who have failed medical therapy or who develop complications such as renal dysfunction.18 There are a host of available procedures, with unique risk/benefit profiles to consider (Table 1).20-22,25-30 Invasive approaches can be broadly classified into five main categories: prostate resection, prostate enucleation, vapourisation, alternative ablative techniques, and non-ablative techniques.15 The procedures are also classified based on their level of invasiveness, i.e. surgical or minimally invasive (as shown in Table 1).
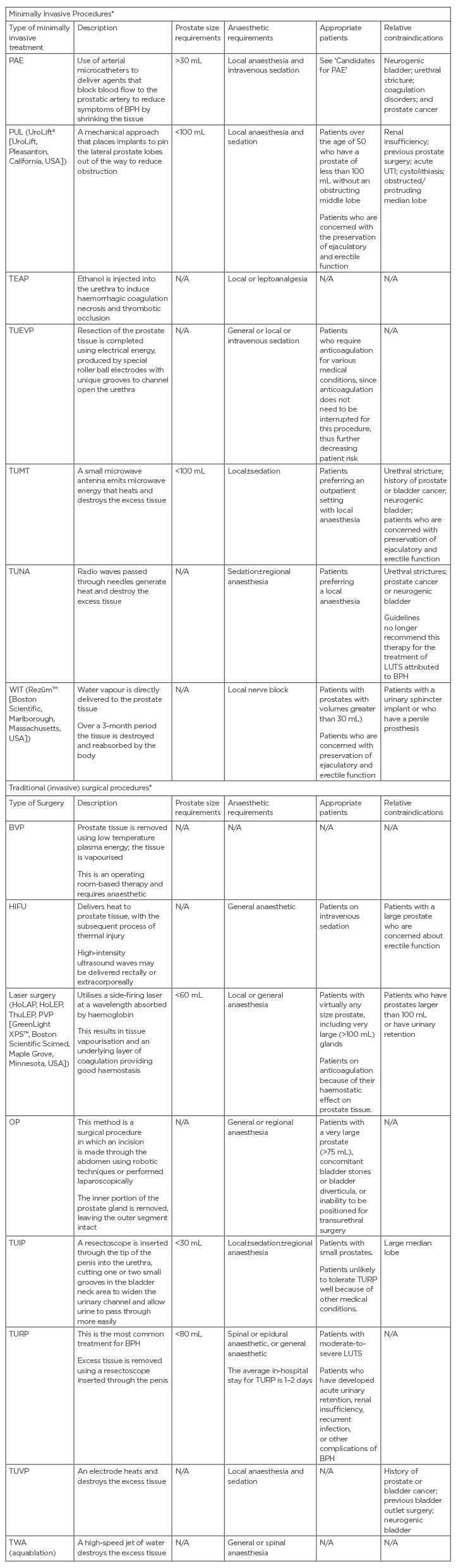
Table 1: Overview of minimally invasive treatments and traditional surgical options for benign prostatic hyperplasia.4,15,18,25-30
*Some of these procedures such as TUNA, TUMT, HIFU, and TEAP are rarely performed in the UK. There are very few procedures done under a true local anaesthetic.
BVP: plasma button electrovapourisation; HIFU: high-intensity focused ultrasound; HoLAP: holmium laser ablation of the prostate; HoLEP: holmium laser enucleation of the prostate; N/A: not applicable; OP: open prostatectomy; PAE: prostatic artery embolisation; PUL: prostatic urethral lift; PVP: photoselective vaporisation of the prostate; TEAP: transurethral ethanol ablation of the prostate; ThuLEP: thulium laser enucleation of the prostate; TUEVP: transurethral electro vapourisation; TUIP: transurethral incision of the prostate; TUMT: transurethral microwave therapy; TUNA: transurethral needle ablation; TURP: transurethral resection of prostate; TUVP: transurethral vapourisation of the prostate; TWA: transurethral water-jet ablation; WIT: water-induced thermotherapy.
Minimally invasive interventions may be further classified into either thermo-ablative or mechanical.26 The common complications for both surgery and minimally invasive treatments are disease progression and urinary retention, which may require more invasive therapy. Other potential complications include bleeding, infection, strictures, incontinence, and sexual dysfunction.22 Some of these procedures such as transurethral needle ablation, transurethral microwave therapy, high-intensity focused ultrasound, and transurethral ethanol ablation of the prostrate are rarely performed in the UK, and very few procedures are performed under a true local anaesthetic.This review article will focus on prostatic artery embolisation (PAE) as a treatment option for BPH.
OVERVIEW OF PROSTATIC ARTERY EMBOLISATION
PAE offers a unique minimally invasive treatment option for BPH (Figure 2). First performed in 2009,4 PAE blocks the blood supply to the prostate with small microspheres, which causes the prostate tissue to shrink and die. It can be performed under a local anaesthetic with or without intravenous sedation, which benefits patients who cannot tolerate general anaesthetic. Commonly, it is performed as a day case, negating the need for hospitalisation.22,31-35
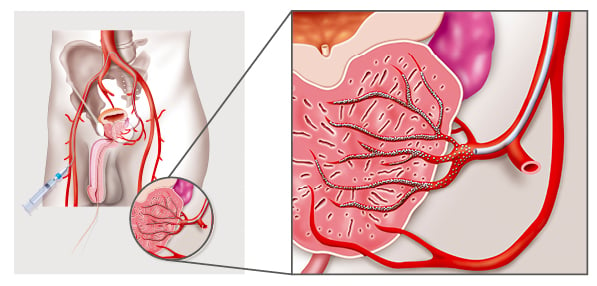
Figure 2: Prostatic artery embolisation, a minimally invasive, non-surgical treatment for benign prostatic hyperplasia.22,31-35
The National Institute for Health and Care Excellence (NICE) has worked with the British Society of Interventional Radiology (BSIR) and the British Association of Urological Surgeons (BAUS) to co-ordinate the United Kingdom Register of Prostate Embolization (UK ROPE) study. The study recruited 305 patients across 17 UK urological or interventional radiology centres, 216 of whom underwent PAE and 89 of whom underwent transurethral resection of prostate (TURP). Patients underwent clinical examinations prior to the PAE procedure and at 3 and 12 months after their procedure. The study found PAE provided a clinically and statistically significant improvement in symptoms and QoL for males with enlarged prostates. The safety profile and quicker return to normal activities associated with PAE was highly beneficial to patients with BPH.35,36 Considering this study and other new evidence, NICE updated its guidance to include PAE as a standard option for the treatment of LUTS related to BPH.22
PAE is an advanced embolisation technique demanding a high level of expertise and should be performed by experienced interventional radiologists who have been trained and proctored appropriately. The use of cone-beam CT is encouraged to improve operator confidence and minimise risks of non-target embolisation. The place of PAE in the care pathway is between that of drugs and surgery, allowing the clinician to tailor treatment to individual patients’ symptoms, requirements and anatomical variation.22,31-35
Candidates for Prostatic Artery Embolisation
Patient selection and meticulous embolisation technique are critical to optimise results with PAE. PAE is a particularly good option for males who are not yet ready to undergo more invasive prostate surgery; in those who have moderate to severe lower urinary tract symptoms and depressed urinary flow due to bladder outlet obstruction; have an enlarged prostate, with no upper limit for the optimal size; have failed medical management of BPH; with repetitive bladder stones or calculi due to outlet obstruction; who wish to maintain sexual function; who are either ineligible or not interested in traditional surgery; or those who are considered not fit for general anaesthesia or are anticoagulated.31 Ineligible patients may include those with advanced atherosclerosis, aneurysmal changes or severe tortuosity in the aortic bifurcation or internal iliac arteries, detrusor failure, neurogenic lower urinary tract dysfunction, urethral stricture, bladder diverticulum, bladder stone, allergy to intravenous contrast media, renal failure (glomerular filtration rate: <40 mL/min/1.73m2), non-visualisation of the prostatic artery or other accessory arteries on CT angiography, coagulation disorders, or on antiplatelet/anticoagulant therapy.14,35,37-42
To ensure optimal results with PAE, the following pre-procedure evaluations may be necessary (Figures 1 and 3):13,22,31-36 urine test for infection; PSA test to exclude prostate cancer; DRE; kidney function (including creatine and estimated glomerular filtration rate); CT or magnetic resonance angiogram; IPSS and International Index of Erectile Function (IIEF) baseline scores; 3-day fluid record; flow rate and residual (including amount passed, speed and time taken); and pressure flow studies. Clinical interpretation of symptom scores, flow test, and PSA results is a complex task that should be carried out by an experienced urologist.
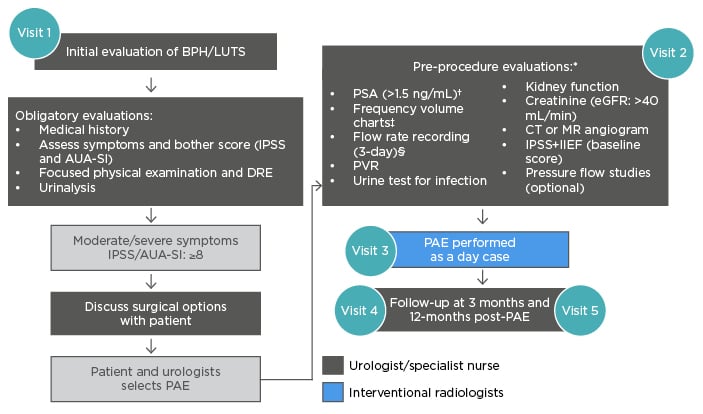
Figure 3: Multidisciplinary workflow for prostatic artery embolisation.17,35,43-47
*In select patients, generally performed by urologist.
†In life expectancy of >10 years, a prostate cancer diagnosis can affect the BPH management plan.
‡Used when nocturia is the primary symptom.
§The FV chart should be 3 typical days, with a flow rate done separately and >1,560 mL to be meaningful.
AUA-SI: American Urological Association Symptom Index; BPH: benign prostatic hyperplasia; DRE: digital rectal exam; eGFR: estimated glomerular filtration rate; IIEF: International Index of Erectile Function; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; MR: magnetic resonance; PSA: prostate-specific antigen; PVR: post-void residual.
What Happens During Prostatic Artery Embolisation?
Under X-ray guidance, the prostatic artery is approached through the femoral or radial artery. Super-selective catheterisation of the small prostatic arteries is performed using fine microcatheters through the pelvic arteries. Once the microcatheter is in place, dye is injected into the vessels to confirm supply to the prostate. Embolisation involves the introduction of microspheres (approved for PAE), which are injected through the catheter and into the prostate blood vessels that supply the gland in order to reduce its blood supply and completely block the vessels. Several embolic microspheres have received approval for PAE. Cone beam CT can be used to also confirm prostatic arterial supply as well as the presence of extra prostatic anastomoses, which commonly occur to the bladder, penis, and rectum. If a position to avoid these anastomoses cannot be achieved, they may need to be coil embolised to prevent non target embolisation.22,31-36,42,48
The outpatient procedure typically ranges from 30–120 min and patients are discharged the same day after a few hours of recovery. Typically, it takes patients a few days at home to fully recover before they can return to work and other routine activities. It is common for patients to experience mild pelvic pain during and after the procedure, which usually subsides after 1–3 days post-procedure. After PAE, the prostate will begin to shrink over the course of the first 3 months. Symptoms usually improve within a few weeks to a few months after the procedure. The procedure is performed in the interventional radiology suite under local anaesthesia and sometimes under conscious sedation. Overnight hospitalisation is rarely required.22,31-36,42,48
Benefits of Prostatic Artery Embolisation
PAE is a unique transarterial option, whereas other procedures are transurethral. Therefore, PAE offers several benefits over other minimally invasive and surgical procedures, including fewer complications and patients avoid the risks of retrograde ejaculation, erectile dysfunction, and sphincter injury associated with surgical treatment. The lower risk of sexual side effects to maintain sexual function is a key strength.50 Urethral catheterisation is not usually needed and there is a low risk of urinary incontinence. The UK ROPE study found the concomitant advantages of reduced length of hospital stay and need for admission after PAE.35,36 PAE may be performed as a day case procedure without a general anaesthetic. It is rare for other procedures to not involve general anaesthesia at least. PAE has a shorter recovery time, minimal blood loss and decreased discomfort. In addition, PAE is associated with QoL improvements and limited side effects compared to surgery.22,31-37,49-51
Potential Risks Associated with Prostatic Artery Embolisation
Patients may experience ‘post-PAE syndrome’ for a few days after the procedure. Most effects are mild, and the risks are lower than surgery. Post-procedure symptoms can include nausea, vomiting, fever, pelvic pain, and painful or frequent urination. Other risks include haematoma at the incision site, blood in the urine, semen, or stool, bladder spasm and infection of the prostate.30-36 The UK ROPE data showed that haematuria and haematospermia were the most common complications, and that haematuria was lower in the PAE (18.6%) compared with the TURP group (63.9%). Haematospermia, however, was more commonly reported by patients in the PAE group (reported by 12.6% of these patients versus 1.6% in the TURP group). Reported retrograde ejaculation rates were lower in the PAE group (24.1%), at approximately half the rate reported by patients in the TURP group (47.5%).35,36 It is important to note that this procedure remains technically challenging due to complex vasculature, anatomical variations and small arteries, with notable radiation exposure levels to both patients and medical staff.22,31-36
Potential Limitations of Prostatic Artery Embolisation
The limited availability of angiographic facilities as well as the restricted number of interventional radiologists may limit the use of PAE. Furthermore, a potential disadvantage of PAE compared with transurethral intervention is the use of radiation during the procedure. The dose can be significant to both patient and operator so it is imperative that techniques to minimise radiation dose are always utilised. Although the radiation dose during a standard PAE procedure do not reach levels of deterministic harm (around 3 Gy), a consideration for any procedure involving radiation are the stochastic effects for patients (chance of malignancy related to the dose). The dose area product per PAE procedure is approximately 17,400 Gy/m2, which corresponds to an effective dose of approximately 47 mSv.52 In a patient population with an average age of 65, this is roughly equivalent to an additional lifetime cancer risk of 0.2% (baseline risk for males is 44.9%). The clinical outcomes are more modest than traditional operations, with some patients deriving insufficient benefit from PAE and ultimately requiring further treatment.52,53
MULTIDISCIPLINARY TEAM MANAGEMENT OF PATIENTS WITH BENIGN PROSTATIC HYPERPLASIA
Historically, BPH was primarily treated by urologists and, as such, many urologists view PAE as a threat and a potential territory invasion. Interventional radiologists have the opportunity to introduce PAE as a procedure that can assist urology with patients who are at high risk for surgery or otherwise problematic with few surgical options available. PAE may be particularly attractive in men with gross BPE for whom a traditional operation could be challenging. Furthermore, guidelines now emphasise a multidisciplinary team approach (Figure 3).54 Shared care between general practitioners, urologists and interventional radiologists in the management of BPH is critical for successful patient outcomes.43 The high incidence of BPH-related LUTS makes it difficult for every individual presenting to be assessed and treated solely by a urologist.44 Despite the efficacy of medical therapy, there will be patients who require referral to a urologist either early on, to rule out prostate cancer and other conditions, or later, after initial medical therapy and lifestyle management has failed.45
In addition, many patients seek out PAE on their own, after research on the internet or word-of-mouth from someone who has undergone the procedure. Referring these patients to urology is an excellent way to further build the collaborative relationship and provides expert discussion of all additional treatment options, and allows the opportunity for urological testing, including uroflows and residuals, PSA level, and full urodynamic testing when indicated. Pre-treatment test results can be complex and require the expertise of a specialist and should, therefore, be carried out by an interdisciplinary team or a urologist.12 Careful patient selection is crucial for PAE and a collaborative decision between urologists and interventional radiologists can aid this selection process. Collaborative working can enable patients to have access to the whole range of BPH treatments and be able to make informed decisions about their treatment. Figure 3 outlines a potential workflow between multidisciplinary team members.
The availability of PAE as a new nonsurgical therapeutic modality is an interesting innovation that offers an enriched therapeutic option. PAE can enable increased patient referrals through collaboration with the interventional radiology team. PAE enables many patients to stop using their medication, with little or no remaining symptoms, and can reduce surgical waiting lists. Building a successful PAE programme requires a joint collaboration to ensure the safety and success of PAE. In addition, effective and regular communication between members is necessary to ensure effective collaborative practice in order to provide high-quality BPH care.
CONCLUSIONS
PAE is a minimally invasive treatment that provides several benefits to improve LUTS caused by BPH. NICE and several other guidelines have decided that the current evidence on the safety and efficacy of PAE for BPH validates recommending it as an effective and reliable therapy.15,19,21,22,49 The UK ROPE study found that PAE provides a clinically and statistically significant improvement in symptoms and QoL for males with an enlarged prostate.35,36 PAE offers several benefits over surgical procedures, including fewer complications, reduced length of hospital stays and shorter recovery time.22,31-36 Medical professionals need to collaborate with urological surgeons and team up with interventional radiologists in order to ensure patients benefit from access to PAE.








