Abstract
Isolated renal hydatid disease, caused by the larvae of the parasitic tapeworm Echinococcus granulosus is a rare phenomenon and accounts for only 2% of all reported cases. The authors report a case of a 12-year-old female who presented with right flank pain. Initial abdominal ultrasound revealed a complex cystic mass in the upper pole of the right kidney. A contrasted CT scan better defined it as a well-circumscribed cyst with multiple thin septations. Laboratory investigations showed eosinophilia and a positive IgG Echinococcus serology. Considering these radiological and laboratory findings, a tentative diagnosis of primary renal hydatid was made. With perioperative antihelmintic therapy, the authors used a combination of an open puncture-aspiration-injection-reaspiration technique pericystectomy to manage the isolated renal hydatid. Renal hydatid can easily be misinterpreted pre-operatively for more sinister renal cystic pathology, including cystic renal cell carcinoma. An accurate pre-operative diagnosis requires a high index of suspicion, especially in endemic regions. Surgical therapy, with perioperative antihelmintic therapy, offers the best chance of cure.
Key Points
1. Echinococcus granulosus is a parasitic tapeworm that primarily affects individuals who live in sheep- and cattle-raising communities through ingestion of infected matter.2. Renal hydatid disease largely affects the liver (75% of cases) and the lungs (15% of cases).
3. Renal hydatid disease can be difficult to diagnose as patients often remain asymptomatic until the cyst enlarges and symptoms begin to present.
INTRODUCTION
Human hydatid disease is caused by the larvae of the parasitic tapeworm Echinococcus granulosus. It is endemic to many sheep- and cattle-raising parts of the world, including the Mediterranean, Africa, South America, the Middle East, Australia, and New Zealand.1 Isolated renal hydatid disease is a rare phenomenon and accounts for only 2% of reported cases.2 The liver and lungs are by far the most common organs affected as these acts as the first two anatomical ‘filters’ of Echinococcus larvae as they enter the portal circulation. The authors report a case of a 12-year-old female with an isolated renal hydatid and review the relevant literature.
CASE PRESENTATION
A 12-year-old female presented to the authors’ department with a two-month history of progressively worsening right-sided flank pain. They reported no dysuria, haematuria, or other urinary symptoms and were otherwise healthy with no previous medical or surgical history of relevance. Notably, the patient lived in an informal settlement in a rural, impoverished area of the province. On clinical examination, their vitals were unremarkable. They only exhibited slight right flank tenderness on palpation, but no apparent masses could be felt. Initial abdominal ultrasound revealed a large complex cystic mass of 5.4×7.0x5.4 cm located in the upper pole of the right kidney (Figure 1). A subsequent abdominopelvic contrasted CT scan confirmed the ultrasound findings and better defined it as a well-circumscribed cyst with multiple thin septations (Figure 2). The cyst did not appear to have any calcifications. The left kidney appeared normal in size and function, and the rest of the abdomen, pelvis, and chest appeared unremarkable. Laboratory investigations showed eosinophilia as well as a positive IgG Echinococcus serology with a weak positive titre of 26. Her renal function and electrolytes were normal, and urine microscopy and culture showed no parasites or small daughter cysts.
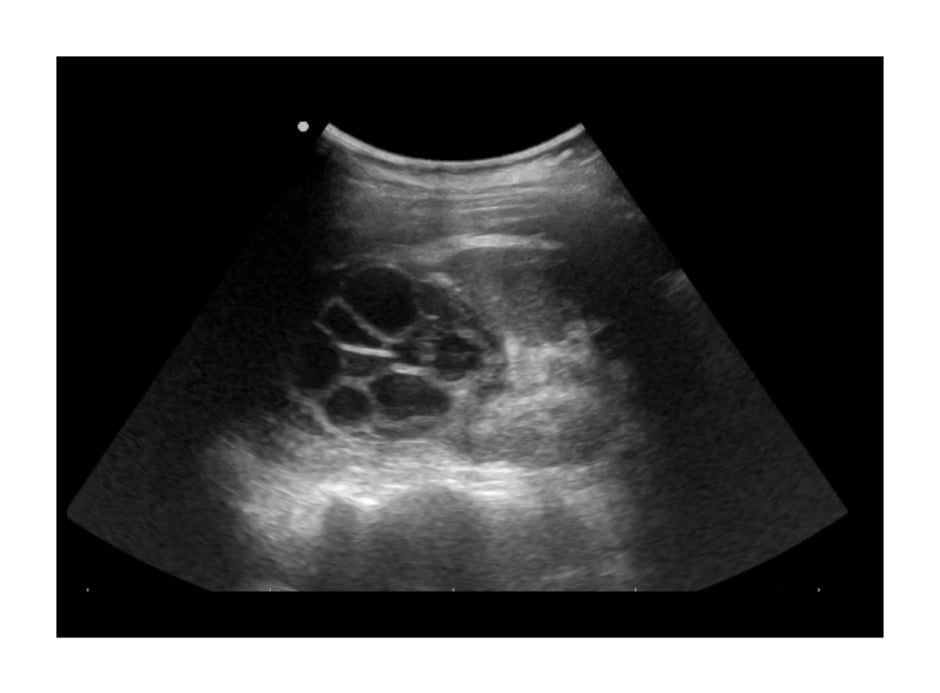
Figure 1: Transabdominal ultrasound findings showing a large complex cystic mass with multiple thin septations in the upper pole of the right kidney.
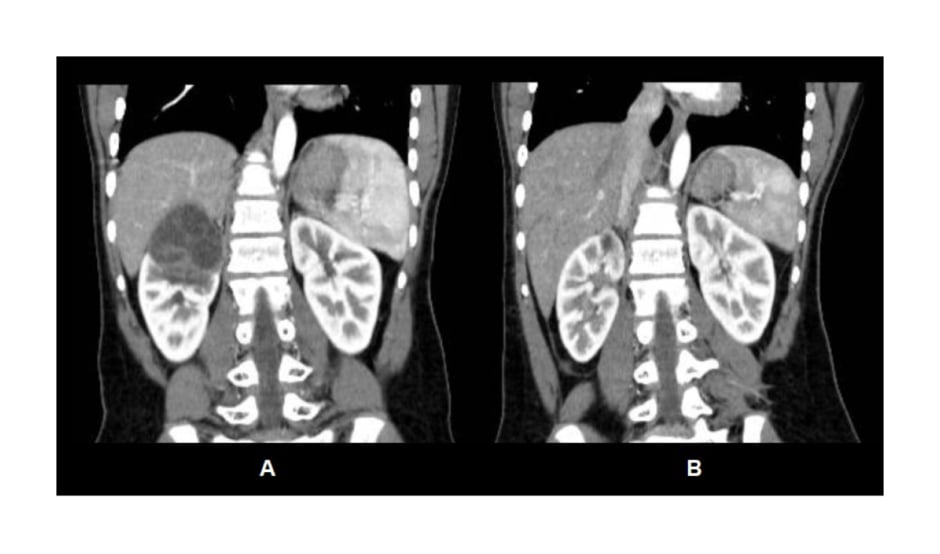
Figure 2: A) Pre-operative contrasted CT abdomen findings showing a well-circumscribed cystic lesion of the right kidney with multiple thin septations. B) Post-operative contrasted CT abdomen demonstrating re-expansion of the right kidney after pericystectomy with minimal scarring of the upper pole.
In light of the positive Echinococcus serology, together with the suggestive CT scan findings, a diagnosis of primary renal hydatid cyst was made. She was initiated on anthelmintic therapy with three cycles of albendazole, with a window of 14 days. Thereafter, the authors performed a right open pericystectomy of the primary renal hydatid through a small extraperitoneal right flank incision. The authors used 20% sodium chloride as an intra-operative scolicidal agent and followed the puncture, aspirate, inject scolicidal agent, re-aspirate (puncture-aspiration-injection-reaspiration [PAIR]) principles before opening the cyst to remove all daughter cysts and entire endocyst (germinal) layer (Figure 3), while packing off surrounding tissues with abdominal swabs soaked in the authors’ chosen scolicidal agent.
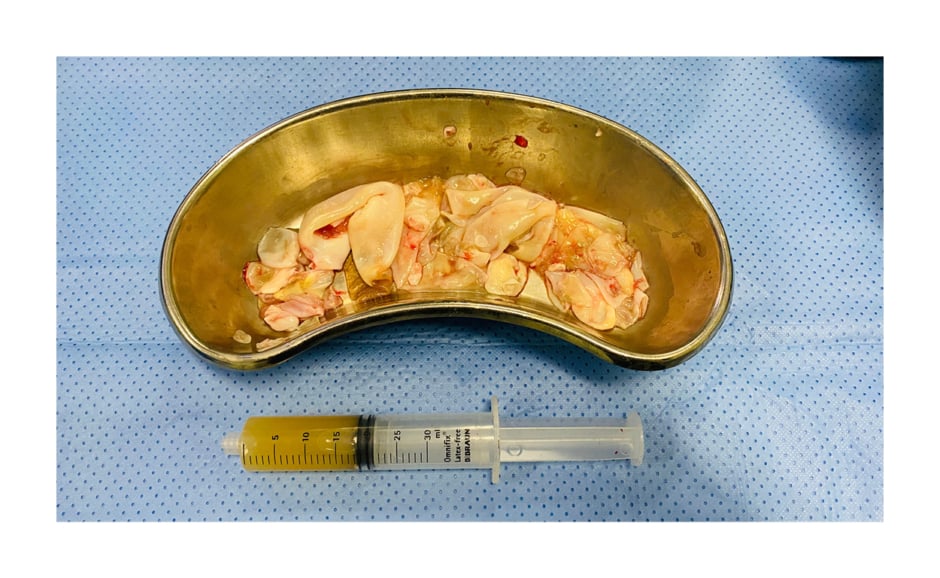
Figure 3: Photograph showing aspirated fluid and multiple collapsed daughter cysts isolated from the cystic lesion.
The cyst cavity was drained overnight with a nephrostomy tube. The drain was noted to have a persistently high output post-operatively. A retrograde ureteropyelography showed a small communication between the cyst cavity and an individual upper pole calyx. A double-J ureteral stent was inserted and drainage from the cyst cavity ceased, the drain was subsequently removed, and the patient was discharged. The double-J stent was removed without complications two weeks after discharge. The patient completed another cycle of oral albendazole post-operatively.
A follow-up CT scan 6 months after surgery (Figure 2) demonstrated re-expansion of the right kidney that was previously compressed by the cyst, with minimal scarring of the upper pole and a normal renal function. The patient will continue follow-up at the authors’ facility to detect for any recurrence.
DISCUSSION
Urogenital hydatid disease is a rare manifestation of hydatidosis, comprising only 2–3% of all cases.3 The kidney is the most common involved urogenital organ but its isolated involvement is an extremely rare clinical entity.4 The parasitic tapeworm E. granulosus, inhabits the small intestines of infected canines, the primary host. Infected parasite eggs are excreted in the stool in large numbers and the intermediate hosts (i.e., sheep, cattle, and goats) then ingest these infected eggs. Humans are infected through direct contact with an infected definitive host or by ingesting contaminated soil, food, and water. The eggs hatch and produce larvae in the small intestine of the host that goes on to penetrate the jejunum and gain access to the venous and lymphatic system of the host, with subsequent spread to distant organs and manifestation as hydatid cysts.1
The liver and the lungs account for most of all cases, 75% and 15%, respectively. Genitourinary involvement, especially isolated renal hydatid disease, is very rare.5 Patients may be asymptomatic for many years before becoming symptomatic due to the progressive enlargement of the cyst.6,7 Symptoms include flank mass, haematuria, vague lumbar or flank pain, and dysuria. Less commonly, renal hydatid disease is complicated by cyst rupture into the collecting system, resulting in hydatiduria,8 which is pathognomonic of renal hydatidosis. Hydatiduria, which is the presence of daughter cysts and larvae (hydatid sand) in urine, is only present in 5–18% of cases and should not be relied upon for a diagnosis to be made.9 Diagnosis is notoriously difficult, unless clinicians keep a high index of suspicion, especially in endemic areas. A combination of clinical history, imaging studies, serological, and urine investigations yields a reliable pre-treatment diagnosis in only 50% of cases, and a presumptive diagnosis in 71%.10 Moderate eosinophilia is a non-specific finding on blood work but is only present in 20–50% of cases.2 Blood serum with the use of indirect haemagglutination, counter immunoelectrophoresis, ELISA, and gold labelled antibody may be used in the diagnosis of human echinococcosis.11 The sensitivity of these tests varies according to the site where the infection is found and the viability of the cyst. In extrahepatic disease, the sensitivity of these tests reduces to about 25–56%, limiting the use of serology to aid in the diagnosis.12
The authors’ patient had a weak positive titre of 26 on Echinococcus indirect haemagglutination serology testing. Renal hydatidosis often has characteristic appearances on imaging modalities. On a plain abdominal radiograph, a ring-shaped calcification can be seen. On ultrasonography, the appearance of a renal hydatid cyst may vary considerably from unilocular cysts that resemble a simple renal cyst to hypoechoic multicystic, or multiloculated cysts with daughter cyst and hyperechoic hydatid sand. As the patient changes position, a ‘falling snowflake pattern’ created by the multiple echogenic foci and produced by the hydatid sand can be seen under real-time imaging. In addition, detachment of the endocyst from the pericyst gives an appearance of ‘floating membranes’ and multivesicular mother cyst with daughter cysts separated by radiating septae representing cyst walls and hydatid sand or matrix may give rise to a ‘wheel-spoke’ pattern.7,13,14 CT is more accurate and sensitive and shows unilocular (Type I), or multilocular cysts (Type II) with mixed internal attenuation and daughter cysts with lower attenuation than that of the maternal matrix, and a completely calcified cyst (Type III).15-17 MRI can delineate the cyst more accurately but offers no advantage over CT, and it is also expensive, therefore it is not routinely used for diagnosis.15
Management options include medical treatment, percutaneous intervention, and surgical treatments. According to the World Health Organization (WHO) guidelines, monotherapy with albendazole is the recommended antihelmintic drug of choice for visceral disease.14,18 Albendazole before surgery, sterilises the cyst by killing the scolices and renders the cyst inactive, reduces cyst wall tension, and reduces the risk of intra-operative cyst rupture.19,20 In addition, post-operative antihelmintic therapy also prevents recurrence. Albendazole (10–15 mg/kg/day) is administered for 1–4 weeks before surgery, and continued for 1–3 months after the procedure is recommended. 14,21 This protocol was followed in the authors’ patient.
The PAIR technique has been described as a safe and effective treatment modality for a renal hydatid.22 Surgical treatment offers the best chance of cure, and nephron-sparing options such as a cystectomy, pericystectomy, and partial nephrectomy should be performed where possible. A simple nephrectomy is indicated for non-functional kidneys. Whatever modality is selected, measures must be taken to prevent intra-operative rupture of cysts and intra-peritoneal spillage, which will result in post-operative recurrence.23 Post-operative antihelmintic therapy, as mentioned above, is also crucial to prevent a recurrence.14,21 The authors’ used a combination of an open PAIR technique with a pericystectomy in view to achieve the best possible result.
CONCLUSION
Renal hydatid disease is an uncommon disease and can be misinterpreted pre-operatively for more sinister renal cystic pathology including cystic renal cell carcinoma. An accurate pre-operative diagnosis requires a high index of suspicion especially in endemic regions. Surgical therapy, with perioperative antihelmintic therapy, offers the best chance of cure and involves completely excising the entire endocyst (germinal layer) with daughter cysts without spillage of the viable cyst contents. It is pertinent that patients are followed up at regular intervals to detect early recurrence of disease, especially if there has been any intra-operative spillage of the hydatid cyst or if the pericystectomy was incomplete.







