Abstract
Penile squamous cell carcinoma (SCC) is a rare malignancy associated with human papillomavirus and immunosuppression. If not detected early in its course, local invasion and metastasis to distant regions often occurs. Colorectal adenocarcinoma (CRAC) is the third most common malignancy worldwide. Many familial genetic mutations are associated with CRAC; however, co-presentation with penile SCC has not been established in literature. The authors present a case in which a patient presenting with a primary diagnosis of penile SCC was found to have distant liver metastases due to a previously unknown recurrence of CRAC. The authors conclude that primary penile cancer with subsequent metastatic colon adenocarcinoma is possible in patients with unknown genetic predisposition.
Key Points
1. Penile squamous cell carcinoma (SCC) is a rare malignancy, presenting in 1% of males, and is associated with human papillomavirus and immunosuppression.2. Many familial genetic mutations are associated with colorectal adenocarcinoma (CRAC), but co-presentation with penile SCC has not been established in literature; this case report outlines a case of concurrent primary penile SCC with a recurrence of CRAC.
3. Genetic mutations that interrupt tumour suppressor genes or mismatch repair pathways could account for a co-presentation of another primary malignancy alongside CRAC.
INTRODUCTION
Penile cancer is rare, representing only 1% of all malignancies in men. Squamous cell carcinoma (SCC) accounts for over 95% of penile cancers.1 Closely-related conditions include Bowen’s disease and Erythroplasia of Queyrat, both of which tend to progress to invasive disease, whereas bowenoid papulosis does not have malignant potential.2 Risk factors for penile SCC include uncircumcised males, human papillomavirus (HPV), immunosuppression, chronic infection, phimosis, poor hygiene, multiple sexual partners, and tobacco exposure.2,3 Diagnosis is delayed in up to 50% of patients due to fear, carelessness, and embarrassment.1,4 This delay often leads to detection of advanced disease, as one-third of patients have progressed beyond organ-confined disease at the time of diagnosis.4 The most common site of metastasis of penile SCC is regional superficial inguinal lymph nodes and then sequentially the deep inguinal and pelvic lymph nodes before visceral spread.1 The staging of SCC based on lymph node involvement is a studied predictor of survivability, with inguinal lymph nodes having approximately 80% survival rates, while pelvic lymph nodes have 0–33%.5 Primary presentation of distant metastasis of penile SCC is rare and occurs late in the course of the disease.4
Colorectal adenocarcinoma (CRAC) is the third most commonly diagnosed cancer worldwide, accounting for 10% of annual cancer diagnoses, excluding skin cancers.6 Risk factors include adenomatous polyps, family history, age, tobacco use, alcohol use, and processed meat intake. Inherited conditions such as familial adenomatous polyposis and Lynch syndrome increase likelihood of a CRAC diagnosis.6 In this case, the authors present a patient without known genetic risk factors diagnosed with primary penile SCC with concurrent metastatic CRAC to the liver.
CASE DESCRIPTION
A 65-year-old male with a past medical history of Type 2 diabetes, hypertension, dilated cardiomyopathy, colon cancer status post-sigmoid colon resection, and end-colostomy 6 years prior to presentation, presented to the emergency department with dizziness and weakness that had lasted for 1 week. The patient also reported penile tension for the past 18–24 months, with associated swelling and bleeding from a circumferential penile lesion. At the time of the patient’s colostomy reversal, a penile lesion was noted, and the patient was referred to the urology department; however, this coincided with the start of COVID-19 pandemic, creating various barriers to follow-up for the patient.
A physical exam revealed skin pallor and a 9 cm erythematous, friable, bleeding circumferential penile mass. There were no palpable inguinal lymph nodes on the physical exam. The patient’s haemoglobin was 5.2 g/dL, necessitating transfusion of two units of packed red blood cells. A CT with intravenous contrast of the abdomen and pelvis detected lymphadenopathy in the left inguinal region measuring up to 1.7 cm, and the right external iliac region measuring 1.1 cm (Figure 1). There were multiple low attenuation foci scattered throughout the parenchyma of the hepatobiliary system. There was a 3.5 cm calcified lesion in the right middle lobe of the liver, as well as two additional 2.7 cm and 1.9 cm calcified lesions in the right posterior lobe and the right inferior lobe, respectively. Evaluation of the gastrointestinal tract was limited during this study given the absence of enteric contrast. The patient was admitted to the hospital for anaemia secondary to a bleeding penile mass.
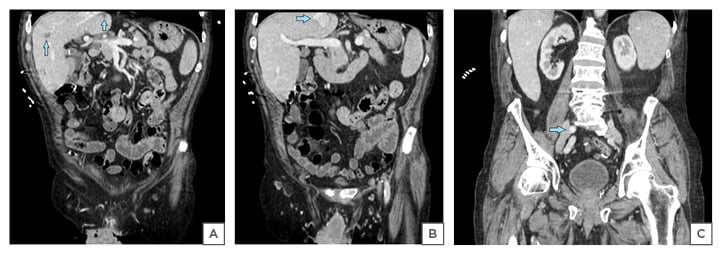
Figure 1: A) Coronal sections of CT abdomen and pelvis showing multiple low attenuation lesions in the liver; B) calcified lesion of liver; C) enlarged right external iliac lymph nodes.
During the hospital course, bleeding was noted from the ventral and dorsal aspects of the penile mass. Bedside interventions to control bleeding were attempted, including application of oxidised regenerated cellulose, pressure dressing with gauze, and silver nitrate; however, haemostasis could not be achieved. Due to the inability to control the bleeding, an additional five units of packed red blood cells were transfused.
A penile biopsy was unable to be performed due to the bleeding risk; therefore, palliative penectomy was elected. The pathologic specimen was an exophytic tumour measuring 9.1 cm x 8.5 cm x 3.8 cm with its papillary surface positive for p16 and p40 markers, confirming invasive, poorly differentiated SCC, classified as an HPV-related, basaloid-type penile intraepithelial neoplasia (Figure 2). Tumour extension was also noted into the scrotum, making the primary tumour Stage pT4. Negative margins of 2 cm were achieved with subsequent pathologic confirmation.
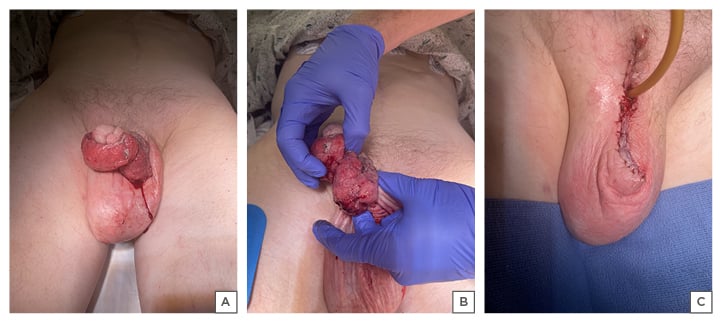
Figure 2: A, B) Circumferential penile mass before; and C) after partial penectomy.
Interventional radiology-guided liver biopsy was performed on the first post-operative day, which revealed metastatic adenocarcinoma inconsistent with a primary source of penile SCC, and more characteristic of a primary tumour of colorectal origin. Immunological stains confirmed that the cells were positive for CDX2 and cytokeratin 20, both markers for metastatic CRAC. Stains were negative for cytokeratin 7 and p40, markers strongly associated with squamous epithelial origin carcinoma (Figure 3).
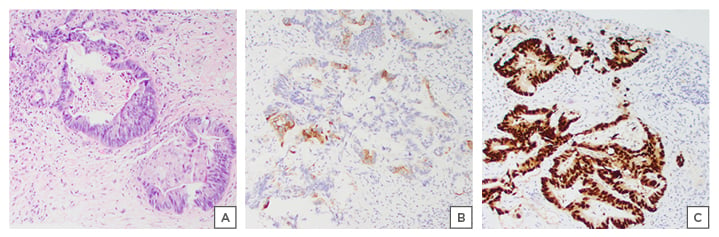
Figure 3: Interventional radiology liver biopsy consistent with A) adenocarcinoma; B) immunological stains positive for CK20; and C) CDX2.
After a liver biopsy, the patient required no further blood transfusions and was discharged on the third post-operative day. In total, they were an inpatient for 13 days. A follow-up with medical oncology recommended an inguinal ultrasound to assess for lymphadenopathy. No palpable inguinal lymph nodes were appreciated during this office visit. Liver involvement from colon cancer was staged at advanced metastatic Stage 4. The disease was noted to be treatable, but not curable. The patient elected to undergo palliative chemotherapy and adjuvant radiation therapy for local control.
DISCUSSION
CRAC and SCC necessitate different treatment modalities and carry different prognoses. It is crucial to ascertain the origin of metastases, especially when dealing with the presence of uncertain metastases, as described in this case.7 While penile SCC itself is rare, concomitant presentation with metastatic CRAC adds another layer of novelty. Initial patient presentation of a circumferential penile mass with hypodense liver lesions suggested the possibility of penile metastasis. Ideally, liver biopsies would have been obtained prior to surgical intervention to confirm malignancy and origin of metastasis. However, in the best interests of the patient, and as a necessary step to control bleeding, palliative penectomy was performed.
After the confirmation of metastatic CRAC to the liver, prophylactic inguinal lymph node biopsy was deferred in the patient. This region of the lymphatic system falls into a potential overlap for the spread of penile SCC and CRAC: penile SCC has predictable lymphatic drainage to the deep inguinal, iliac, and pelvic lymph nodes prior to systemic spread.8 Conversely, CRAC has the tendency to spread haematogenously to liver, lung, and brain,5 but there has been reported literature of skip metastasis of CRAC to inguinal lymph nodes and external iliac lymph nodes.9 The decision not to perform lymph node biopsy leaves an unanswered question to this case: were pelvic lymph nodes enlarged due to spread of penile SCC, CRAC, or reactive? According to the National Comprehensive Cancer Network (NCCN) guidelines for penile cancer,10 enlarged pelvic lymph nodes are recommended to be biopsied if feasible. If the lymph nodes are involved, chemoradiotherapy is recommended in patients who are non-surgical candidates.10 In the setting of Stage 4 metastatic colon cancer, an interdisciplinary decision to start chemotherapy for metastatic disease superseded the inguinal lymph node biopsy.
Penile cancer is rare in the general population. Risk factors for penile cancer are HPV, lack of circumcision, and genetic mutations.2 Histological evaluation of the penile specimen classified the sample as HPV-related basaloid intraepithelial neoplasia. HPV is a common, often asymptomatic infection, and it is a reasonable hypothesis that at some point, the patient may have acquired an undocumented HPV infection which predisposed them to later developing penile SCC.
In contrast to penile SCC, CRAC is a common malignancy that has both genetic and behavioural risk factors, including alcohol consumption.11 The patient had ongoing alcohol abuse, with documented consumption of up to 28 standard drinks per week. While ethanol itself is not carcinogenic, once oxidised, its acetaldehyde intermediate can exert carcinogenic effects by binding DNA through deterring replication and mitosis, and can even serve as a photosensitiser upon binding DNA. While this is a known risk factor for CRAC, reports in literature also link alcohol intake with SCC risk.12 Also, recent evidence suggests that males who drink alcohol are 2.3 times more likely to develop inguinal lymph node metastases from penile cancer than those who do not (considered less than eight drinks per year).13 The patient’s high alcohol consumption, plus the ambiguity of return of the CRAC, may have contributed to an immunosuppressed state, leading to increased susceptibility for SCC.
A genetic aetiology for the two aggressive malignancies is another consideration that cannot be overlooked. Due to the frequency and aggressiveness of documented colonic polyps on a previous colonoscopy, as well as the patient’s paternal history of CRAC diagnosis in his 60s, genetic screening of a colorectal cancer panel was performed. This panel tested for well-documented mutations associated with early-onset CRAC (CDH1), Lynch syndrome (MLH1, MSH2, MSH6, EPCAM), familial adenomatous polyposis (APC, AXIN2, NTHL1), MSH3-associated polyposis (MSH3), MUTYH-associated polyposis (MUTYH), juvenile polyposis syndrome (SMAD4, PTEN), Li-Fraumeni syndrome (TP53, CHEK2), hereditary mixed polyposis syndrome (BMPR1A, GREM1), polymerase proofreading-associated polyposis syndrome (POLD1, POLE), and Peutz–Jeghers syndrome (STK11). Many of these conditions have interruption of tumour suppressor genes or mismatch repair pathways, which could account for a co-presentation of another primary malignancy along with CRAC.
The patient was negative for mutations in all of the genes mentioned. While a genetic aetiology for this rare presentation would have been the answer to multiple malignancies in this patient, the absence of these mutations does not rule out a genetic cause. It is possible that the patient has an unknown mutation that has yet to be characterised. There may be complementary mutations, or additional environmental or epigenetic factors that could explain the aggressive CRAC and co-occurrence with penile SCC. Without targeted therapy options for germline mutations, treatment options with palliative chemotherapy without curative intent were pursued.
CONCLUSION
The authors conclude that concomitant penile cancer and metastatic colon cancer can occur in patients without significant risk factors. The patient in this study was circumcised and negative for well-described germline mutations. The aetiology of aggressive malignancy in this patient is unclear. While it is not uncommon to see metastatic disease at the time of presentation of penile SCC, it is a rare occurrence, and not previously reported in the literature when those metastases are found to be associated with a primary tumour other than that of the presenting penile cancer.








