Abstract
Overactive bladder (OAB) is clinically defined as the presence of urinary urgency and may be associated with diurnal urinary incontinence, frequency, and enuresis, and/or constipation. In children aged 5–10 years, the prevalence is 5–12%. Association with emotional disorders is widely described in the literature. Constipation is associated with voiding symptoms because of crosstalk between the gastrointestinal tract and the urinary tract. OAB is believed to be multifactorial. Correct functioning between the pontine micturition centre, the periaqueductal grey matter, anterior cingulate gyrus, and prefrontal cortex is important for correct voiding development and the process of maturation. Patients with OAB have greater anterior cingulate gyrus activity and deactivation of the pontine micturition centre urinary inhibition process, leading to a greater frequency of bladder repletion sensation. Urotherapy is the first treatment to be initiated and aims to change behavioural patterns inthese patients. Other treatment options are anticholinergics, with oxybutynin being the most widely studied, but also described is the use of tolterodine, darifenacin, and mirabegron. Alternative treatments, such as nerve stimulation in the parasacral or the posterior tibial area, have shown improvement of symptoms in comparative studies with conventional drug treatment, and, in refractory cases, botulinum toxin A is an option. In this article, we review the pathophysiology, associated conditions, and aspects related to diagnosis and treatment of OAB.
INTRODUCTION
Overactive bladder (OAB) is a common lower urinary tract dysfunction in children, defined as the presence of “urinary urgency, usually accompanied by frequency and nocturia, with or without urinary incontinence, in the absence of urinary tract infection or other obvious pathology.”1 Clinically, urgency is commonly associated with daytime urinary incontinence and frequency; however, it may be due to voiding postponement and bladder overdistension.2,3
OAB is not associated with neurological and anatomical alterations of the lower urinary tract. The overall prevalence ranges from 1.5–36.4%; it is known that the peak incidence occurs between 5 and 7 years of age, with a higher prevalence in males.4,5 The quality of life of these patients may be associated with emotional and behavioural changes, such as symptoms of depression and anxiety. Patients may have problems such as attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder.6-8
Although OAB can resolve itself spontaneously, urinary symptoms can persist into adulthood.9,10 The correlation between the voiding and bowel symptoms is already very established. Children with OAB have three times more symptoms of constipation than children without urgency.11-13 The possible explanations are that the intestinal and bladder functions are controlled by the supraspinal region,14-17 and there seems to be crosstalk between the lower urinary tract and the rectum (Figure 1).18 Moreover, it has been suggested that contractions of the external urethral sphincter to prevent urinary loss and simultaneous contraction of the anal sphincter may, by negative feedback, reduce the rectal transit.14,18 Also, the hard mass of stool in the rectum could compress the bladder and modify its function.19-21 Obese children have a higher incidence of OAB when compared to eutrophic children. Studies suggest that there may be a frontal lobe disinhibition, justifying this phenomenon.14,15,22 Children with sickle cell and institutionalised children also have a higher prevalence of OAB.22 This article will review the pathophysiology, associated conditions, and aspects related to diagnosis and treatment of OAB.
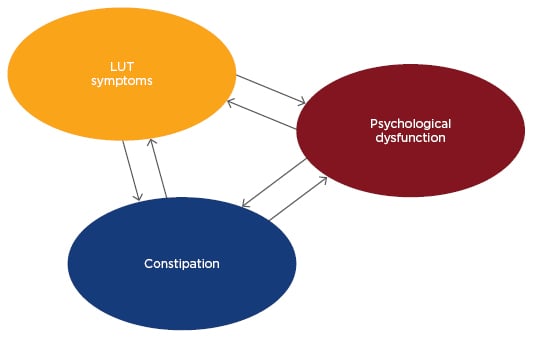
Figure 1: Multifactorial pathophysiology of bladder, bowel, and brain dysfunction.
LUT: lower urinary tract.
PATHOPHYSIOLOGY
The pathophysiology of OAB is not completely understood and is believed to be multifactorial. One theory is that the urgency and related symptoms stem from a cortical immaturity of the centres responsible for controlling urination.14 Supporting this hypothesis, voluntary and co-ordinated urination is developed over time. In the first 2 months of life, the child voids once per hour with an intermittent flow. In this phase, the voiding is mainly controlled by the brainstem.19,23 From the first to the third year of life, cortical inhibitory pathways and the pontine micturition centre along with periaqueductal grey matter, anterior cingulate gyrus (ACG), and the autonomic, somatic, and sensorial autonomic nervous systems are being developed, and urination becomes voluntary.19,23 With the child’s development, the prefrontal cortex would typically start to have top-down control over more primitive afferent pathways of the brain, such as the limbic and paralimbic system.
The child may also experience voiding urgency by deciding not to urinate as part of a behavioural dysfunction. Similarly to lack of attention, voiding postponement may be the result of a behavioural dysfunction or neurophysiological immaturity. Children with oppositional defiant disorder who have difficulty following commands often refuse to go to the toilet to void or defecate, and may be affected by functional faecal incontinence.24,25 Unfortunately, little is to be found in the literature on the neurofunctional assessment of these patients, but there seems to be hypoactivation of the prefrontal cortex.26-28 In situations of voiding postponement, the child engages in techniques such as tightening the penis, which stimulates the dorsal nerve of the penis and thus causes the external urethral sphincter to contract and the bladder to relax.
Patients with OAB seem to have a greater ACG activity and a deactivation of the pontine micturition centre urinary inhibitory process, leading to a higher frequency of bladder repletion sensation.3 Therefore, a hyperactive ACG may lead to a greater responsiveness to bladder filling and, consequently, to urgency.
CLINICAL HISTORY AND DIAGNOSIS
The clinical history should include family history, neuropsychomotor development, voiding and bowel training, urinary tract infection (UTI) history, school performance, and the child’s behaviour and psychosocial development.23 It was recently demonstrated that there is an association between mothers and daughters with OAB.10,29 The intestinal constipation should be investigated and evaluated through the Bristol Stool Diary and the Roma III or IV score.11
OAB occurs during the bladder storage stage. However, it may be accompanied by changes in the voiding phase, such as dysfunctional voiding. In a study, the authors demonstrated that some urinary symptoms have a low correlation with more objective data.30 Therefore, additional exams should be requested, such as urine tests, ultrasound (US) of the urinary tract, uroflowmetry, a bladder diary, and a bowel diary. The US should measure the post-void residual (PVR) and the bladder wall thickness and evaluate the rectal distension (≥3 cm suggests faecal impaction).11 The PVR is different according to age. The PVR is considered high in children 4–6 years old if it is ≥30 mL or >21% of bladder capacity, and in children 7–12 years old if it is ≥20 mL or >15% bladder capacity.2
A patient with OAB has a bell-shaped or tower-shaped uroflowmetry curve, which suggests urinary urgency (Figure 2).1 A urodynamic study should be reserved for those cases where there is failure after treatment or for patients who have signs of non-neurogenic bladder or even myogenic failure.23,31
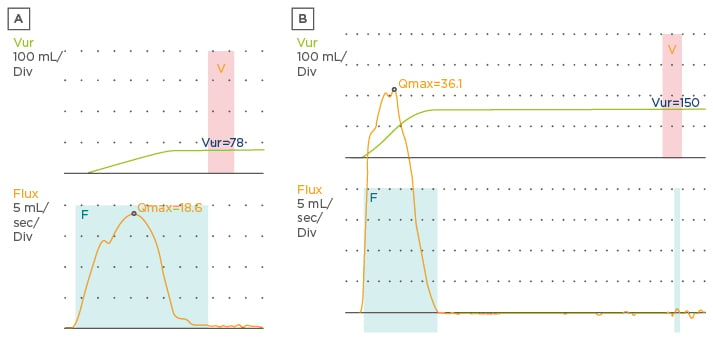
Figure 2: Uroflowmetry curves. A) Bell-shaped curve; B) tower-shaped curve.
The bladder diary consists of the record of frequency and urine volume and of daytime urinary incontinence for a period of 48 hours. The expected bladder capacity is calculated by [Age (years) + 1] x 30 mL. The International Children’s Continence Society (ICCS) defined as increased daytime urinary frequency as ≥8 times per day and decreased daytime urinary frequency ≤3 times per day.2 The bowel diary is made using the Bristol Stool Scale, also for a period of 7 days. Tools suchas the Dysfunctional Voiding Symptom Score (DVSS), the Vancouver Symptom Score for Dysfunctional Elimination Syndrome (VSSDES) questionnaires, and ROME III and IV should be used. The VSSDES can indicate diagnostic validity. The cut-off point is 11 with a threshold diagnostic sensitivity and specificity of 80% and 91%, respectively.32 In the DVSS questionnaire, levels above six for girls and nine for boys are considered suggestive of lower urinary tract dysfunction.33
TREATMENT
Standard Urotherapy
Urotherapy is a very important measure in the treatment of OAB and is considered the first line of treatment. It consists of dietary hygiene changes regarding fluid intake, an adequate interval between urinations, adequate positioning to void, and defecation where the feet are supported on a flat surface with attention to the perineal musculature so that there is co-ordination between pelvic floor relaxation and bladder contraction.11 Caffeine, chocolate, and citrus foods can be avoided because they may stimulate the urgency symptom, although there is a low level of scientific evidence for these measures.3,5,11,23,34 The approach to constipation should be aggressive through hydration and fibre intake. We treat all constipated children with polyethylene glycol (PEG 3350). Initially, we treat faecal impaction at a dose of 1.0–1.5 g/kg/day (maximum dose of 100 g/day) for at least 2 months. The child should be encouraged to stay in the bathroom for 15–20 minutes at regular times.11
Anticholinergics
Anticholinergics are the first line of medical treatment for children who maintain OAB after urotherapy.3 All anticholinergics may have side effects, such as dry mouth, constipation, blurred vision, urinary retention, plethora, dizziness, and delirium, especially at high doses. Oxybutynin is the most common drug and can be given as either immediate release (IR) or extended release (ER) with a recommended dose of 0.3–0.6 mg/kg.5 van Arendonk et al.35 retrospectively analysed 27 patients with daytime urinary incontinence who used IR oxybutynin and switched to slow ER oxybutynin. A total or partial improvement of symptoms was observed in 13 of the 27 patients with an increased voided volume (33% versus 53%; p<0.01) and an improved bladder capacity (55% versus 70%; p=0.03).35 Intravesical administration is an alternative that allows for higher doses, with fewer adverse effects. However, this method is of little use because the children do not undergo intermittent catheterisation.34 Tolterodine is also available in two formulas, and some studies suggest a good efficacy and good tolerability in children.36,37 Bolduc et al.36 demonstrated that 77% of the children using tolterodine because of non-tolerability of oxybutynin proceeded without significant side effects. Tolterodine IR is available in doses of 1 mg or 2 mg, while ER is 2 mg or 4 mg. Studies comparing the results of oxybutynin and tolterodine in children with OAB are limited; however, they suggest that tolterodine is at least as effective as oxybutynin.38,39 Fesoterodine is the most recent long-acting antimuscarinic and is available in 4 mg or 8 mg. It is recommended for children weighing >25 kg and it is well-tolerated by children and adolescents.40 Solifenacin is a slow-acting antimuscarinic available in a dose of 5 mg or 10 mg. Hoebeke et al.41 demonstrated a total response rate of 85% and presence of adverse effects in 6.5% of patients with the use of solifenacin. Darifenacin is an antimuscarinic with a more selective action with less adverse effects on the central nervous system.5 A combined therapy of two antimuscarinics (such as oxybutynin and tolterodine) is also an option; a previous study showed that the combined drugs showed an improvement in urinary continence, and 63% of patients had moderate adverse effects, but not enough to stop the medication.41
Mirabegron is the β3 agonist approved for treatment of OAB in adults and has few studies of its use in children.5 It increases bladder capacity without changing the bladder pressure and PVR and works by promoting smooth muscle relaxation through the increase of cyclic adenosine monophosphate.3 The adverse effects commonly found in antimuscarinics have not been demonstrated in mirabegron.5 Blais et al.42 conducted a study in 58 children with refractory OAB and treated them with mirabegron for 11.5 months. They demonstrated a statistically significant improvement in bladder capacity and continence in these patients, with moderate adverse effects in 13% of the cases. This medication may be used as an adjuvant medication to another anticholinergic if necessary.11
Neuromodulation
There is a hypothesis that electrical neural stimulation (ENS) stimulates the nerves of the bladder, spinal cord, and central nervous system. The mechanism of action is still uncertain. There may be a local effect with activation of the bladder C fibres, or a spinal effect with sympathetic activation and/or parasympathetic inhibition.43 Dasgupta et al.44 conducted positron emission tomography/computed tomography (PET/CT) in eight patients with Fowler’s syndrome and eight healthy controls to map the brain function of these patients during full bladder and the modulation during sacral nerve stimulation. In healthy controls, there was an increase in midbrain function and chromogranin A during full bladder. The Fowler’s syndrome patients had no increase in brainstem activity, despite the increase in chromogranin A function in the absence of neuromodulation. The hypothesis is that neuromodulation assists in the re-establishment of voiding desire and the voiding capacity of patients.44
The main types of ENS are a) parasacral transcutaneous electrical nerve stimulation (TENS);3,5,43,45 b) posterior tibial nerve stimulation (PTNS);3,4,43,46 and c) stimulation by sacral implantation.3,43 In the TENS, two surface electrodes are placed on S3 level, whereas in the PTNS a 34-gauge needle is placed two fingers above the medial malleolus. Quintiliano et al.47 carried out a review study in which patients undergoing ENS showed complete resolution of OAB symptoms, urgency, and daytime incontinence in 31–78%, 25–84%, and 13–84% of patients, respectively. Randomised studies have shown TENS to be an effective method in the resolution of OAB symptoms in children and adolescents.47,48 The rate of complete resolution of symptoms with TENS is around 60%, with a recurrence rate of 10%.43,46
Borch et al.49 randomised 51 patients to three treatment groups. Group 1 received TENS plus active oxybutynin administration, Group 2 received TENS plus placebo oxybutynin administration, and Group 3 received placebo TENS plus active oxybutynin administration. All three groups experienced a positive effect in terms of decreasing urinary incontinence, incontinence severity, and urinary frequency, and improving maximum and mean bladder capacity, but in Group 3 one-third of the patients had urinary retention, which did not happen in Group 1; this signalled the importance of TENS in bladder emptying. Group 1 had a more significant improvement for daytime urinary incontinence (p<0.01), severity of urinary incontinence (p<0.05), and urinary frequency (p<0.001) when compared to the other groups.49
More recent studies have compared established treatments for OAB and TENS. Quintiliano et al.47 analysed two methods for the treatment of OAB in children: a) oxybutynin plus sham scapular electrical therapy, and b) TENS plus placebo. They demonstrated that TENS is as effective as oxybutynin for the treatment of OAB. In the oxybutynin group, there was a decrease in voiding frequency, but the TENS group showed an improvement in constipation. Adverse effects were only present in the oxybutynin group and resulted in treatment discontinuation in 13.3% of the patients.47
TENS can also be used in the treatment of constipation. Veiga et al.50 demonstrated that TENS resolved 60% of cases of constipation in patients with OAB, although it was unrelated to any improvement in OAB. Two other randomised studies have already been completed. In one study, the participants who received 20-minute TENS sessions demonstrated increased colonic transit time compared with a control group.51 The second study was a double-blind, controlled study that evaluated the acute effects of TENS on rectal motility and it showed that after TENS the bowel contractions improved.52
The PTNS approach is well tolerated in children, and studies have shown that it is an effective method in the treatment of OAB.53 Initial studies have demonstrated an improvement in complete resolution of OAB symptoms by 56–100%.5,54 The rate of complete resolution of symptoms was significantly higher in the TENS group compared to the PTNS group (70% versus 9%).55 Boudaoud et al.56 analysed PTNS versus sham treatment and concluded that, despite the improvement of urodynamic parameters in the PTNS group, there was no difference in clinical results between the two groups. Peters et al.57 conducted a randomised, multicentre study comparing PTNS and tolterodine ER and found a similar reduction in urinary frequency, severity of urgency, and increased voiding volume.
We recommend ENS in cases of standard urotherapy failure because there are no direct side effects and a higher effectiveness against the OAB and constipation. The sacral implant is performed through percutaneous transforaminal access to stimulate the S3 nerve.3,43 Roth et al.58 demonstrated complete or >50% improvement in 88% of children. Of these, 63% had nocturnal enuresis, 89% improved daytime urinary frequency, and 59% had constipation.2 However, because of the high rate of reoperations, this method is limited to patients who are refractory to the other methods.
Botulinum Toxin A
Botulinum toxin A acts in the neuromuscular junction preventing the action of acetylcholine and adenosine 5-triphosphate on the parasympathetic presynaptic terminations, promoting chemically reversible desensitisation and flaccid paralysis of the muscle.45 The effect begins 5–7 days after injection and can last for about 6 months. The botulinum toxin A injection is a viable option for patients with refractory OAB, but few studies have been performed for non-neurogenic patients. Hoebeke et al.59 reported a study with 21 children with non-neurogenic OAB in which 100 U of botulinum toxin were given at 15 injection sites in the bladder wall. Few adverse effects were reported, with a complete success rate of 42% in the first application, a 61% increase in bladder capacity (p<0.001), and 11% recurrence within 1 year.59 It should be used in children ≥3 years of age at the dose of 5–10 U/kg. It is contraindicated in patients with peripheral motor neuropathy, neuromuscular junction disorders, UTI, uncorrected coagulopathy, and in pregnant women. The adverse effects are pain, UTI, haematuria, and autonomic dysreflexia. Around 2–9% of patients may present postprocedure transient acute urinary retention requiring clean intermittent catheterisation at this stage.5
CONCLUSION
OAB is a common clinical entity in children and knowledge of the neurophysiology factors of voiding provides information that helps to manage it. The diagnosis is clinical and should be complemented with a bladder diary, US of urinary tract with PVR evaluation, and uroflowmetry. Treatment should start with urotherapy, and in refractory cases TENS, PTNS, and medications can be included. If the symptoms persist, botulinum toxin A and sacral neuromodulator implants may be alternatives.








