Abstract
Anterior urethral strictures affect the male urethra between the tip of the penis and the apex of the prostate. These form the bulk of urethral strictures in men. The common causes for urethral strictures seem to be idiopathic or related to instrumentation of the urethra. Clinically, patients have varying obstructive symptoms associated with the progressive narrowing of the urethral lumen. Treatment modalities have aimed at incising or excising the fibrous tissue, augmenting the damaged area by grafts or flaps, or more recently, replacing the area with tissue engineered constructs. As the biology of wound healing and fibrous tissue formation is not yet completely understood, urethral strictures continue to pose a challenge to clinicians and scientists.
INTRODUCTION
Urethral stricture disease (USD) affects 0.6% of the at-risk male population.1 Patients present with symptoms such as difficulty in voiding, nocturia, and painful urination, resulting in significantly reduced quality of life.2 The underlying causes and rates of incidence depend on patient age, race, geography, and socioeconomic status.1,3-5 Conventional treatments, such as dilatation, urethrotomy, and urethral stent, aim to reverse the progressive narrowing of the lumen. Surgical repair with a buccal mucosal urethroplasty has emerged as a gold standard over the years, with a success rate as high as 95%. However, the procedure is associated with limitations, such as donor site morbidity, longer surgical times, and the recurrence of urethral strictures.6 Therefore, efforts to deploy acellular scaffolds and tissue engineered urethral substitutes have been made.7-28
Firstly, this review aims to outline the epidemiology, aetiology, and pathophysiology of USD. Secondly, conventional methods of USD management are discussed from a clinician’s point of view. Lastly, we highlight the state-of-the-art in tissue engineering-based urethral substitutes and outline the potential of tissue engineering in urethral reconstruction surgery.
EPIDEMIOLOGY
The real incidence of urethral strictures is not known, but estimates can be obtained from representative population datasets. Incidence varies based on age, race, geography, and socioeconomic status. The incidence is 1.6 and 10-times higher in older versus younger patients in the USA and UK, respectively.1,3 Beyond the age of 65 years, incidence steadily increases, peaking with men >85 years old.4,5 In the USA, African-Americans and Hispanics have a higher incidence of urethral strictures in comparison with Caucasians.1,5 Urethral strictures are 2.6-times more prevalent in urban centres than in rural ones. In developing countries, the prevalence of urethral strictures is thought to be much higher because of higher rates of infectious and inflammatory strictures,4,29 and they typically affect a much younger population.4,30,31
AETIOLOGY OF URETHRAL STRICTURES
The four main aetiologies of urethral stricture are trauma, inflammation, iatrogenic, and idiopathic.32 In developed countries, these strictures account for 15%, 19%, 33%, and 33% of all strictures, respectively.32 Trauma to the anterior urethra in the form of blunt straddle injury occurs predominantly in the bulbar urethra, resulting in urethral strictures.33,34 In developed countries, the most common cause for inflammatory strictures is lichen sclerosus (LS), which accounts for approximately 5–14% of urethral strictures.3,35,36 Iatrogenic strictures are caused typically due to instrumentation and tend to occur frequently in the bulbar urethra.37 The occurrence of idiopathic strictures is predominantly in the bulbar urethra (88.4%),37 and is more frequent in younger patients.3,38 Idiopathic strictures may be due to unrecognised childhood trauma, as it may take years before the manifestation of significant strictures.39,40 Understanding the aetiology is important as it impacts the stricture location and the success rate of reconstructive surgery.
PATHOPHYSIOLOGY OF THE URETHRAL STRICTUR
Urethral stricture is a fibrotic process initiated by urethral mucosal injury. Epithelial ulceration exposes the corpus spongiosum, leading to spongiofibrosis and resulting in poorly compliant tissue and a diminished urethral lumen.41 Urethral strictures are characterised by significant changes in the extracellular matrix of the spongiosum, such as an increased number of myofibroblasts, increased collagen deposition, and reduced elastin content.42-44 The degree of injury and underlying aetiology of the urethral stricture determines the extent of spongiofibrosis.
DIAGNOSIS AND EVALUATION OF URETHRAL STRICTURES
Urethral stricture patients may experience a steady deterioration of the urinary stream, concomitant with lower urinary tract symptoms, such as urinary hesitancy, incomplete bladder emptying, and nocturia.2,45 Clinical assessments should include history, a clinical examination of the genitalia, and an ultrasound evaluation of the urethra and bladder. Clinically, one can recognise the lichen sclerotic changes, scars, and changes related to previous repairs.46 A retrograde urethrogram (RUG) provides clinically relevant information concerning the location, length of the stricture, and any associated pathologies.45 The sensitivity of RUG in the assessment of urethral strictures is approximately 75–100% with specificities in the range of 72–97%.47 However, RUG does not allow for direct examination of the spongiofibrosis and relies on the examiner to conclude its presence on the basis of intraluminal data.47 Ultrasound may be more sensitive compared with RUG in determining the stricture length and the degree of spongiofibrosis.47 Cystoscopy is considered the most specific test to diagnose urethral obstruction.47
MANAGEMENT OF URETHRAL STRICTURES
The principle of treatment of urethral strictures is to restore and maintain the luminal diameter for as long as possible. This can prevent obstructive symptoms caused by progressive urethral narrowing, and the complications associated with residual urine. The current treatment modalities available for the management of urethral strictures include dilatation, urethrotomy, and urethroplasty.
Dilatation
Urethral dilatation dynamically extends the urethral lumen by means of dilators that are calibrated in accordance with the French system, in which the dilator size correlates with the urethral circumference in millimetres.2 Dilators vary from metallic dilators to flexible plastic or polyurethane dilators. The complications associated with dilatation include injury, bleeding, false passage creation, inadequate dilatation, and stricture recurrence.48 Dilatation may exacerbate spongiofibrosis and therefore, it is not recommended for strictures caused by LS. However, some patients who are not suitable for urethroplasty or reluctant to undergo the procedure may prefer self-dilatation with flexible dilators. This can be continued as long as patients do not suffer from complications. At present, the use of metal dilators is limited to the dilatation of the meatal stenosis and submeatal strictures. Repeated dilatation can make future surgical repair more difficult and less successful.49
Urethrotomy
During urethrotomy, an incision is made through the stricture to the healthy underlying tissue to increase luminal calibre.50 The incision can be made in a blind fashion using an Otis urethrotome, or a direct vision internal urethrotomy (DVIU) with a cold knife or a laser. Urethral tissue tearing is the main risk associated with using the Otis urethrotome. The complications of DVIU include bleeding, bacteraemia, false passage creation, meatal stenosis, extravasation of fluid into the spongiosum, urinary sepsis, and erectile dysfunction. Strictures with the most promising response to DVIU are short (<1 cm) bulbar urethral strictures with minimal fibrotic narrowing of the lumen. Several methodshave been tried to improve the outcomes of urethrotomy. Laser urethrotomy has been attempted, but has not shown superior outcomes when compared to cold knife urethrotomy.51 Intralesional injection of medications such as corticosteroids,52 Mitomycin C,53 and intraurethral Captropril gel54 have been used in an attempt to decrease the fibrotic response after DVIU. No long-term follow-up data are available to determine the true benefit of such strategies. Studies have reported leaving the in-dwelling catheter for 1–4 days.55 However, prolonged duration of the in-dwelling catheter has not reported any superior benefits.
DVIU can be supplemented with the placement of either Wallstent™/Urolume® permanent stents or the Memokath™ temporary stents. The long-term success rate of stents is 85%.56 The complications associated with stents are stent migration, stenosis, urethral obstruction, and the need for reoperation. Urethral stents are indicated for patients with short (<3 cm) bulbar urethral strictures who are unfit for urethroplasty.56
Long-term cure by DVIU is not likely after the third instance of incision/dilatation or in cases where stricture recurrence occurs within 3 months of the first incision. At present, DVIU is used as a maintenance treatment.
Urethroplasty
Urethroplasty is considered the ideal treatment of anterior urethral strictures. The types of urethroplasty available include excision and primary anastomosis (EPA), augmentation and substitution with a dorsal or ventral onlay graft, or a flap. Stricture length, location, pliability of the urethral plate, and lumen of the stricture area dictate the type of urethroplasty. Urethroplasty with the exception of the EPA can be carried out as a single stage or multiple stage procedure, depending on the amount of healthy tissue available at the time of surgery. In order to devise the surgical strategy, anterior urethral strictures can be divided into simple strictures which include strictures of the mucosa with or without spongiofibrosis, of idiopathic aetiology, and complex strictures which include strictures due to LS and failed hypospadias repairs.
Excision and primary anastomosis
EPA is the surgical reconnection of the ends of the urethra after resection of the fibrotic tissue in between. The long-term success of EPA for short (<2 cm) bulbar urethral strictures is around 90–95%, therefore it is recommended for such strictures regardless of aetiology or prior treatment.57 Complications of EPA include fistula, urinary tract infection, post-micturition dribble, and erectile dysfunction.57 Incomplete stricture excision and mobilisation of urethra may result in the failure of the treatment.58
Augmentation and substitution urethroplasty
For strictures >2 cm in length, the anastomosis is augmented using a buccal mucosa graft (BMG) placed ventrally or dorsally, with a tissue flap if necessary. The BMG is usually obtained from the inside of the cheek, the inferior surface of the tongue, or the inner surface of the lip. Donor site morbidity associated with graft harvesting includes oral numbness and restricted movement of the mouth.6
In substitution urethroplasty, the strictured portion of the urethra is replaced with grafts or flaps. Several autologous grafts or flaps from genital and extra-genital skin or mucosa have been used, but BMG is the most popular choice because of ease of graft harvest and surgery. The functional outcomes of skin and BMG are comparable.59
However, in the case of LS related strictures with autoimmune aetiology, BMG is the recommended graft because surgical reconstruction using genital skin tends to end in failure. In the case of complex anterior strictures of LS, on the basis of the urethral plate and the extent of luminal obliteration, a 1-stage (Kulkarni or Asopa technique) or 2-stage Johansen procedure is carried out. Dubey et al.60 showed good results using this 2-stage technique: 22 of 25 patients (88%) had successful outcomes at a mean follow-up of 32.5 months. Kulkarni et al.61 corroborated these results, reporting a 91% success rate at a mean follow-up of 38 months.
Complications of urethroplasty include post-void dribbling, diverticulum/pouch, urinary tract infection, chordee, urethrocutaneous fistula, impotence, and reoccurrence of strictures.62,63 In spite of high overall success rates, meticulous urethroplasty technique, and good substitution material, stricture recurrence has been reported. After substitution urethroplasty, the recurrence has two marked features: fibrosis of the grafted area and fibrous ring strictures at the anastomotic sites.63 We have a hypothesis to explain this based on our experience with tissue engineering. Upon anastomosing two edges of the tissues, the actual closure happens due to the multiplication of the basal epithelial cells. Therefore, the other layers of the graft and recipient area are not involved in multiplication and become redundant, thereby giving rise to an inflammatory reaction. In the process of expelling the non-multiplying cells, the subsequent inflammation could lead to fibrosis. In cases of hypospadias repair failure ending in strictures, the urethral tube lacks the support of the spongiosum, resulting in stricture recurrence.
In the past, urologists believed in treating urethral strictures progressively starting from simple procedures moving towards complex treatment options even though repeated unsuccessful attempts at less invasive procedures can make future surgical repair more difficult.64 Though urethroplasty has a 95% success rate, many urologists have little experience performing this procedure, resulting in their preference for repeated endoscopic procedures in spite of unsatisfactory results.
QUALITY OF LIFE OUTCOMES
The success rates associated with the surgical techniques employed to treat urethral strictures have been well-documented. However, the outcomes relating to the patient’s quality of life, including sexual function, are less widely known and reported. Temporary erectile dysfunction following anterior urethroplasty is a known complication and may occur in up to 38% of men, with the highest incidence of erectile dysfunction following bulbar urethroplasty.65 However, patient ejaculatory function is less well-documented. Validated instruments to define and document patient related outcomes and quality of life are necessary to obtain a good measure of success following reconstructive surgery.
TISSUE ENGINEERED CONSTRUCTS IN URETHRAL RECONSTRUCTION
Tissues currently used for urethroplasty lack the biochemical, mechanical, structural, and/or functional properties of the native urethra and are associated with complications, such as donor site morbidity, rejection, and/or suboptimal performance. However, tissue enginnering has demonstrated promise in developing tissue subtitutes that can restore urethral function in the form of acellular matrices as well as cell-seeded tissue engineered constructs.
A number of acellular extracellular matrix-based scaffolds have been used to treat urethral strictures, as summarised in Table 1.7-21 The efficacy of the acellular grafts is dependent on the extent of vascularisation of the graft and regeneration of epithelial mucosa by infiltration of epithelial cells from adjacent areas. In patients with severe spongiofibrosis and patients with long strictures, acellular grafts do not perform well due to the lack of vascularity and offer only limited epithelial regeneration.17,19 Epithelial regeneration has been demonstrated in a maximum length of 0.5 cm, thus limiting acellular graft application.6
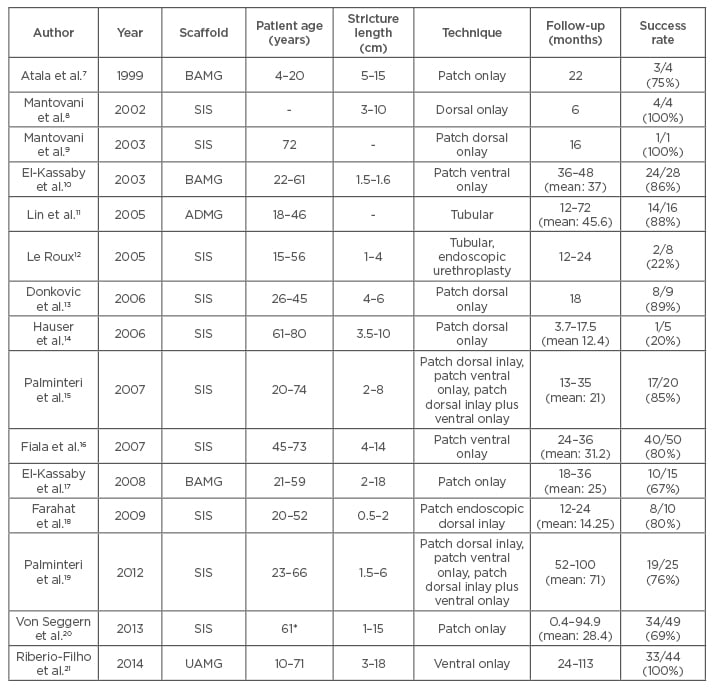
Table 1: Acellular scaffolds used in treating urethral strictures.
*mean value.
BAMG: bladder acellular mucosal graft; SIS: small intestine; ADMG: acellular dermal matrix graft; UAMG: urethral acellular matrix graft.
Tissue engineered cell-seeded grafts may be ideal for long segment and complex strictures as they may succeed in lengthening the distance over which epithelial regeneration occurs. Despite significant progress in developing novel cell- seeded constructs for urethral substitution and subsequent success in preclinical studies, very few have progressed to clinical studies (Table 2). Five patients with LS strictures underwent urethroplasty with oral epithelial and oral fibroblast seeded de-epidermised dermis.23 One patient required full graft excision and another required a partial excision due to fibrosis during short-term follow- up. After 9 years, four out of the five patients still have patent and normal urethras.26
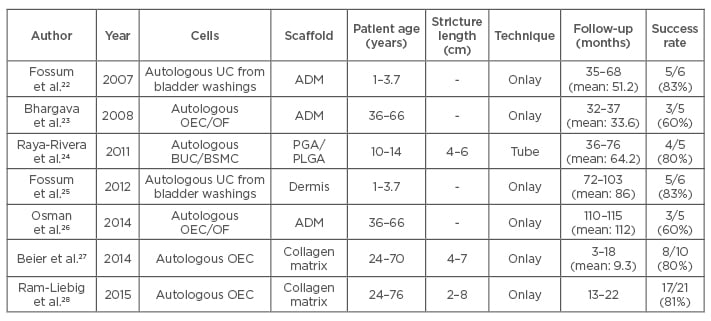
Table 2: Cell-seeded constructs used in treating urethral strictures.
ADM: acellular dermal matrix; UC: urothelial cells; OEC: oral epithelial cells; OF: oral fibroblast; BUC: bladder urothelial cells; BSMC: bladder smooth muscle cells; PGA: polyglycolic acid; PLGA: polylactic-co-glycolic acid.
Six hypospadias patients were treated with acellular dermis scaffolds seeded with urothelial cells obtained from bladder washes.22 One patient developed a stricture and two developed fistulae which were surgically corrected. However, long-term follow-up showed that four out of six patients in this study had a bell-shaped urine flow rate curve with no evidence of stricture or fistula.25 Five paediatric patients were treated with bladder derived urothelial cells and smooth muscle cell seeded polyglycolic acid: polylactic-co-glycolic acid scaffolds which were tubular in shape.24 At a mean follow-up of 71 months, the success rate was 100% with all patients showing the maintenance of patent urethra without strictures. MukoCell® is a commercial tissue engineered collagen matrix seeded with autologous oral epithelial cells available exclusively to patients in Germany. MukoCell was used to treat 10 patients with a success rate of 80% after a mean follow-up of 9.3 months.27 Strictures reoccurred in two patients within the first 3 months. The success rate of MukoCell in 21 patients was 81% after a mean follow-up of 18 months.28 All the above studies demonstrate the feasibility and efficacy of using tissue engineering based approaches for the treatment of complex urethral strictures.
There are several challenges associated with developing tissue engineered therapies for urethral reconstruction. A significant challenge is the non-availability of large animal models which mimic the pathophysiology of USD to evaluate the tissue engineered urethral substitutes. These models need to be developed in order to obtain reliable data about the safety and efficacy of novel tissue engineered substitutes. Another challenge lies in the sourcing of cells. Currently, tissue engineered urethral substitutes have primarily focussed on repairing or replacing the epithelial cell layer in the urethral lumen. However, in the case of severe spongiofibrosis, an efficacious urethral substitute must contain epithelial cells in the lumen in conjunction with endothelial cells and corporal smooth muscle cells in order to repair the damaged corpus spongiosum. In such an approach, the donor source for these cell types is a significant challenge. Finally, tissue engineered products are also met with challenges concerning regulatory issues, and high development and manufacturing costs. Tissue engineered products are positioned to enter the market within a landscape of strict legal regulation and guidelines concerning patient safety. Therefore, the onus of adhering to strict rules lies on all scientists and clinicians, which may limit the possibilities of carrying out specific research. For a typical tissue engineering product, a minimal research and development time of 5 years with a concomitant investment of more than €10 million is necessary.67 In addition to manufacturing (which is currently very expensive) costs include characterisatoin of materials and culture media, and safety and efficacy testing, thereby limiting the development of substitutes.
FUTURE
Urethral stricture has been a complex problem from time immemorial. New technologies and techniques have enabled urologists to provide patients with prolonged periods of non-obstructive urine flow. Tissue engineering, on the other hand, has been able to provide different materials to improve the success rate with decreased morbidity. However, the knowledge of the underlying mechanism of USD remains limited and therefore, USD remains a challenge to conquer for clinicians worldwide.








