Abstract
Renal angiomyolipoma is a benign tumour characteristically composed of fat, smooth muscles, and thick-walled blood vessels. In extremely rare instances, transformation into the more sinister leiomyosarcoma causes potentially drastic differences in both management strategies and prognosis. In most cases, radical nephrectomy remains the standard modality of choice for these tumours as they exhibit aggressive biological behaviour with unfavourable prognosis. Herein, the authors describe a rare case of renal angiomyolipoma in a 33-year-old male, which showed malignant transformation into leiomyosarcoma 5 years post angioembolisation and was managed with radical nephrectomy.
Key Points
1. Renal angiomyolipoma (AML), although a benign entity, can transform into leiomyosarcomas, significantly altering prognosis and creating a need for more aggressive treatment, including surgical resection alongside additional adjuvant therapies.2. This case report describes an unusual clinical scenario in which renal AML underwent a malignant transformation in a 33-year-old male 5 years post-angioembolisation and was managed with radical nephrectomy.
3. Sarcomatoid transformation of renal AML, although rare, can occur after angioembolisation. Due to its malignant potential, patients should undergo regular follow-up for early detection and timely intervention to improve outcomes.
BACKGROUND
Sarcomatous transformation of the angiomyolipoma (AML) is a rare event with a handful of cases reported in the literature.¹ It is marked by rapid growth, early metastasis, and cachexic symptoms.1 The exact cause and duration after which such a malignant change occurs is unknown. On the other hand, angioembolisation has recently become the mainstay of managing patients with AML, especially after rupture with stable haemodynamics, or in patients with syndromic associations, such as tuberous sclerosis (TS), where these tend to be bilateral and multiple. It is a common practice not to intervene surgically, with patients kept on radiological follow-up in the absence of well-defined advantages of the surgical intervention. Herein, the authors discuss a patient with TS and bilateral AMLs who underwent angioembolisation of a ruptured AML in the right kidney and presented to them 5 years later with a rapidly growing mass at the same site. They were found to have leiomyosarcoma of the embolised AML. The authors also conducted a review of the literature of all reported articles on sarcomatous change of the AML, and to the best of the author’s knowledge, such delayed transformation of a post-embolisation AML into a leiomyosarcoma has never been reported. This report underscores the need and importance of regular, time-bound radiological follow-up in patients who undergo angioembolisation to ensure optimal outcomes.
CASE PRESENTATION
A 33-year-old male with a known case of TS (history of epilepsy and facial angiofibroma) presented to the emergency department in 2019 with signs of shock, right flank pain, and anaemia. After resuscitation, a contrast-enhanced computed tomogram (CECT) abdomen was obtained, revealing multiple fat-dense lesions (-10HU) suggestive of angiomyolipoma in both kidneys with intralesional pseudoaneurysm in the segmental branch of the right renal artery and 19×15 cm perirenal haematoma with mass effect over the parenchyma (Figure 1A–1B). Given the presence of multiple AMLs and the goal of preserving kidney parenchyma, surgery was deemed unnecessary. Instead, the patient underwent a successful glue embolisation and was discharged the following day in stable condition. Afterward, the patient was lost to follow-up and remained symptom-free for the next 5 years, without receiving any adjuvant treatment of any sort for those 5 years. However, after 5 years, the patient presented with malaise and loss of appetite and weight. A CECT of the abdomen revealed a 12×11 cm heterogeneous, avidly enhancing lesion, with prompt washout in the venous phase in the upper pole of the right kidney with blurred margins along the adjacent liver, as well as a 24 mm radiodensity at the midpole depicting postembolisation glue with a reported final impression of renal cell carcinoma (Figure 1C–1D). Given the significant increase in lesion size and cachexic symptoms, indicating sarcomatous change or new malignancy, an 18-fluorodeoxyglucose positron emission tomography (18F-FDG PET-CT) scan was performed. This showed an FDG-avid, ill-defined lesion in the upper pole of the right kidney having maximum standardised uptake value (SUVmax) of 5.3, with another mildly FDG-avid lesion in the right thigh muscle. A core needle biopsy was performed, which suggested a low-grade spindle cell lesion, likely benign. A preliminary diagnosis of RCC was made, and the patient underwent a right open radical nephrectomy with an uneventful postoperative course and discharge on the Day 7. Histopathological evaluation of the mass revealed all three hallmark diagnostic criteria of leiomyosarcoma; that is, the presence of spindle-shaped cells arranged in interlacing fascicles, bundles showing moderate to marked pleomorphism including the presence of multinucleated giant cells with brisk mitosis (>10/10 high power field), and necrosis involving <50% of the tumour mass (Figure 2A–2C). The tumour was breaching the renal capsule and extended into the hilar fat; however, resection margins of the renal vessels and the ureter were free. Hilar lymph node resected en bloc was also positive for similar histology. On immunohistochemistry (IHC), tumour cells were diffusely positive for the smooth muscle markers vimentin and smooth muscle actin (SMA), suggesting leiomyosarcoma. Interestingly, the histology also revealed focal presence of HMB-45 in the background, a marker that is usually absent in primary leiomyosarcoma, suggesting a malignant transformation of the earlier angiomyolipoma into its more sinister malignant counterpart (Figure 2D–2F). After a multidisciplinary discussion, the decision was made not to initiate any adjuvant therapy, considering the patient’s intermediate Federation Nationale des Centers de Lutte Contre le Cancer (FNCLCC) grade. Instead, it was agreed to proceed with a 6-monthly follow-up for ongoing surveillance. During the follow-up in the 5th month, 18F-FDG PET CT showed no evidence of residual or recurrent tumour. The patient was doing well at the 6-month follow-up.
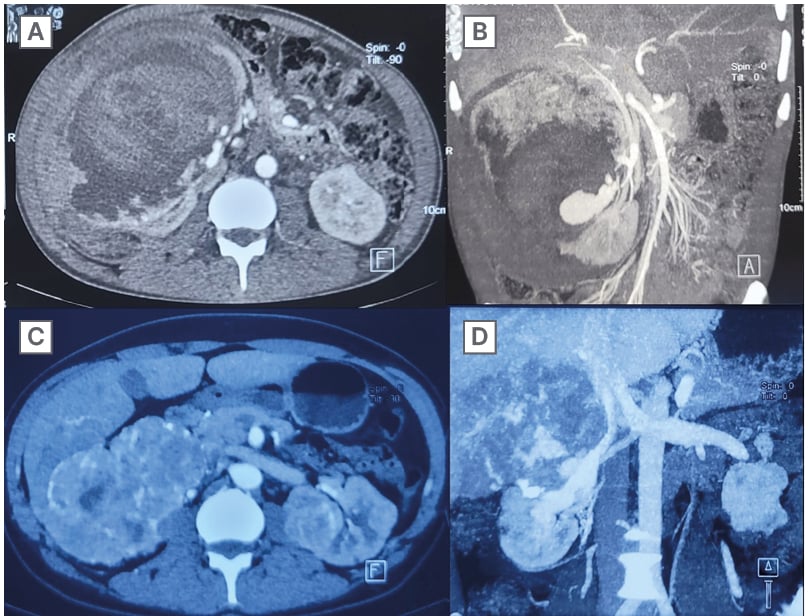
Figure 1: CT images depicting renal lesions over respective timelines.
A and B) CT images descriptive of ruptured right AML with large perinephric haematoma compressing the renal parenchyma.
C and D) CT images depicting heterogeneously enhancing, infiltrating mass from the right kidney with indistinct margins with liver and IVC.
AML: angiomyolipoma; IVC: inferior vena cava.
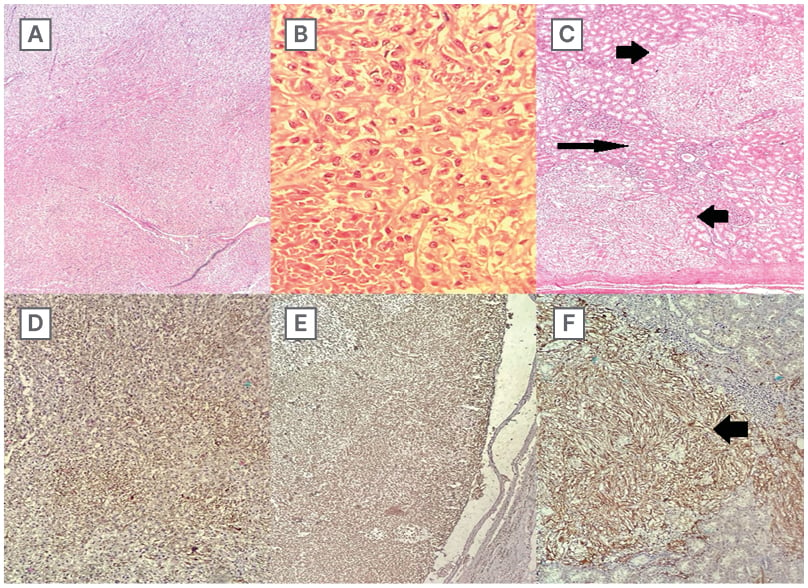
Figure 2: Histology of tumour mass.
A) Interlacing fascicles of neoplastic cells along with branching slit-like blood vessels at low power.
B) Brisk mitosis and necrosis were observed.
C) Pockets of tumour deposits were seen invading the renal parenchyma, tumour nodules (thick arrows), and normal parenchyma (long arrow).
D) Tumour cells were diffusely positive for vimentin.
E) Tumour cells exhibited focal positivity for SMA.
F) HMB showed focal positivity in nests and islands of tumour deposits infiltrating the renal parenchyma, brown-coloured tumour nodule suggesting HMB45 positivity (thick arrow).
HMB: human melanoma black; SMA: smooth muscle actin.
DISCUSSION
Renal angiomyolipoma usually has a benign course, and treatment is based on the lesion size, symptoms, and presentation. TS-associated AML tends to be multicentric and bilateral and has a higher likelihood of progression, whereas sporadic AML more commonly presents as a solitary lesion.2 Observation and selective embolisation are the mainstays of management options, while surgical excision is reserved for selective cases. Patients who undergo selective embolisation seldom undergo consolidation surgery, even when the embolisation is for a large or ruptured AML. The literature indicates that 85% experienced post-embolisation syndrome, with re-embolisation or surgical intervention needed in 14% and 16% of patients, respectively.2
Sarcomatous change in AML is a rarity, with a handful of cases reported in the literature. The authors conducted a literature review with 13 case reports, including three diagnosed post-mortems (Table 1A–1B).1,3-14 The most common presenting complaint was flank pain (54%), followed by haematuria (23%) and incidental diagnosis (23%). All the patients were primarily managed surgically, with 54% receiving some sort of adjuvant treatment during the postoperative period. However, the prognosis remained dismal in most of the reports. To date, no genetic mutations have been identified that link the sarcomatous transformation of AML, which has precluded the development of targeted therapies. Furthermore, the epithelioid variant of AML has aggressive characteristics with a high potential to become malignant, and it has been suggested to be treated in line with renal cell carcinoma.15
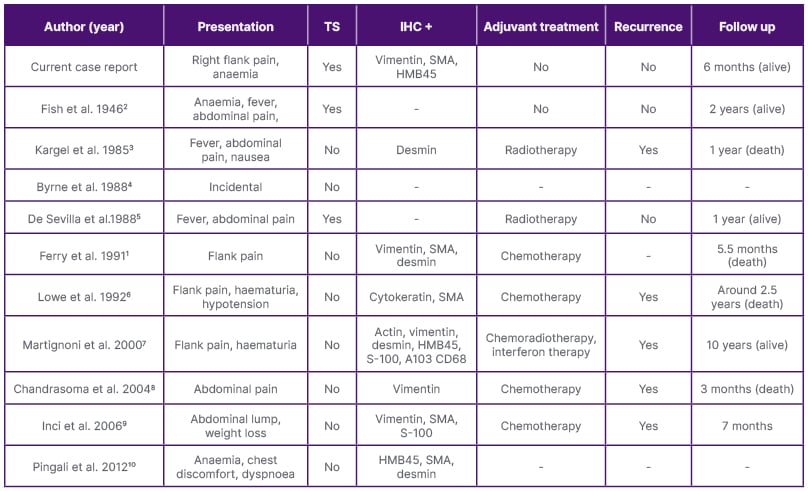
Table 1A: Comparison between data obtained from the authors’ review and other case reports with respective
follow-up and adjuvant therapy.
CD 68: cluster of differentiation 68; HMB45: human melanoma black–45; IHC: immunohistochemistry; A103: melan A monoclonal antibody; SMA: smooth muscle actin; S-100: soluble in 100% ammonium sulphate; TS: tuberous sclerosis.
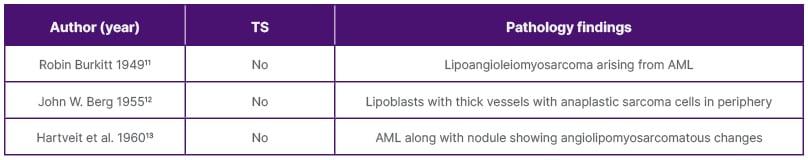
Table 1B: Case reports with diagnosis on autopsy findings.
AML: angiomyolipoma; TS: tuberous sclerosis.
In the author’s case, either there was a transformation of the embolised AML into leiomyosarcoma or de novo development of leiomyosarcoma synchronously with the AML, with possible likelihood of the former, as supported by IHC expression of vimentin and SMA in the background of human melanoma black-45 (HMB45), suggesting that the sarcoma originated from the same site as the original AML. To the author’s knowledge, there have been no reports till now, that an AML can undergo sarcomatous change despite embolisation and is of clinical significance as embolisation is being offered more commonly and surgical consolidation is seldom performed. AMLs with sarcomatous change have metastatic potential and tend to perform poorly.
There is no data on whether the outcomes of post-embolisation sarcomatous transformation are similar to those of de novo sarcomatous transformation, but it is safe to assume, that the possibility of a sarcomatous change in the long term, no matter how small, merits regular follow-up scans to assess for the development of the same at the very least and curative surgical options should be offered at the earliest. Follow-up protocols tend to vary but typically involve cross-sectional imaging, such as CT or MRI, conducted every 1–2 years to detect any potential transformation.
CONCLUSION
Sarcomatoid transformation of renal AML is an extremely rare encounter and can possibly occur despite angioembolisation. Given its malignant potential and dismal prognosis, patients who undergo embolisation should be kept on regular follow-up to facilitate early detection and optimal management of sarcomatous transformation.
Consent for Publication
Informed consent for publication was obtained from the patient, ensuring their identity remains confidential. While we have the patient’s permission to share their case, all identifying information has been omitted to protect their anonymity and privacy throughout this article. This approach upholds ethical standards while contributing valuable insights to the field.







