Abstract
In recent years, immunotherapy has been proposed for the treatment of asymptomatic or minimally symptomatic metastatic castrate-resistant prostate cancer (PCa). Clinical trials using Sipuleucel-T have demonstrated a survival benefit in PCa patients, suggesting that this cancer is linked to a limited immune response. However, the outcome of PCa treated with immune therapeutics has limited benefits in monotherapy: novel vaccination approaches and immune checkpoint blockade gave disappointing results. Several combinations of therapies, such as novel cancer vaccines or checkpoint inhibitors with different immunotherapeutic agents, combined with hormone therapy (enzalutamide, abiraterone acetate), radiotherapy or radium-223, DNA-damaging agents (olaparib), or chemotherapy (docetaxel) hold great promise for eliciting an immune response and improving clinical outcomes in PCa. The goal of immunotherapy is to overcome immunosuppression and destroy cancer cells, or at least to induce those pathways that go back from ‘the escape phase to equilibrium phase’ according to the definition of cancer immunoediting. The aim of this review is to analyse the immune responses during PCa progression and to present the current data regarding immune therapies for PCa.
INTRODUCTION
Prostate cancer (PCa) is the most common malignancy diagnosed and the second most common cause of cancer-related death among men worldwide.1 Androgen deprivation therapy (ADT) is the primary therapeutic approach, but, despite the high rate of progression-free survival (PFS), 30–50% of patients progress to castration-resistant prostate cancer (CRPC), detected by the rising of prostate specific antigen (PSA) values in the blood.2
CRPC presents a spectrum of diseases, ranging from the asymptomatic form without evidence of metastasis, to the advanced form with multiple distant metastases (mCRPC) and with a poor prognosis. CRPC is characterised by hyper-activation and/or over-expression of the androgen receptor (AR) resulting in the transcription of downstream target genes and consequent tumour progression, notwith standing the presence of castrate levels of circulating testosterone in patients.3 mCRPC patients are currently treated with chemotherapeutic drugs, such as docetaxel and cabazitaxel, or with the new generation of anti-androgens, enzalutamide and abiraterone acetate, which target AR activation either directly or indirectly.2 In 2013, radium-223 dichloride was U.S. Food and Drug Administration (FDA)-approved for the reduction of bone metastases.4 Targeting the immune system represents an important tool for patients with this form of disease and combined therapies with multiple immunotherapies or with immunotherapy and conventional compounds are currently under evaluation.
There is a continuous crosstalk between tumour cells and the immune system during cancer progression; an anti-tumour immune response is activated both by local tissue damage and by mutated proteins expressed by cancer cells. On the other hand, tumour microenvironment alters myeloid and lymphoid cells, facilitating the progressive suppression of the host immune response.5,6
Several studies show that the adaptive immune system recognises PCa tumour-associated antigens (TAA), as demonstrated by the presence of tumour infiltrating lymphocytes (TIL) and auto-antibodies to prostate-specific proteins in the peripheral blood of patients.7 A high amount of TIL has often been correlated with longer patient survival, whereas a decrease in TIL was detected in high grade prostatic adenocarcinomas compared to benign nodular prostatic hyperplasia.8 Certain immunosuppressive phenotypes are associated with a moderate recognition of PCa antigens, which drives tumour progression. Moreover, M2-polarised tumour-associated macrophages (TAM) represent a significant component of PCa inflammatory infiltrate, and a high density of TAM is observed in both epithelial and stromal compartments of tumour tissue and is statistically associated with poorer prognosis.9 Increased number of TAM in PCa biopsy is predictive of worse recurrence free survival in men treated with primary ADT. Regulatory T cells (Tregs) and/or myeloid-derived suppressor cells are also increased in the tumour tissue and peripheral blood of PCa patients, and this feature correlates with other negative prognostic factors, such as lactate dehydrogenase, alkaline phosphatase, PSA, and anaemia.10 Finally, a strong correlation between the presence of dendritic cells (DC) and PCa prognosis has been reported, since metastatic patients show fewer circulating myeloid DC than their age-matched controls and a lower number of DC correlates with a higher Gleason score, while DC are elevated in low risk cancer.11 The recruitment of mesenchymal stem cells also supports an immunosuppressive microenvironment,12 as well as elevated levels of indoleamine 2, 3-dioxygenase, nitric oxide, IL-2, prostaglandin E2, transforming growth factor-β, arginase, and adenosine.6
The goal of immunotherapy is to overcome the immunosuppressive microenvironment and to stimulate an immune response against tumour cells to improve patient outcomes. On the other hand, it is important to evaluate how much the immunological system is compromised when considering the most appropriate immunotherapy.13 Herein, the authors analyse the different approaches of immunotherapy for PCa, used both as a single-agent or in combination. Table 1 contains a list of the ongoing studies mentioned in this article.
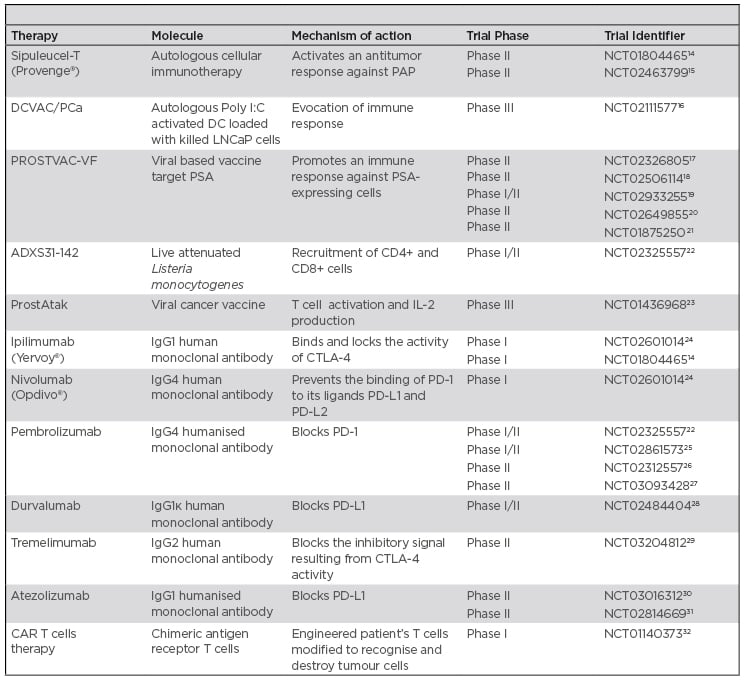
Table 1: Summary of mentioned ongoing clinical trials in prostate cancer.
CANCER VACCINES
The goal of cancer vaccines is to induce the immune system to recognise TAA and to elicit a T cell response aimed at reducing tumour mass and protecting against tumour recurrence or metastatic disease. Some prostate-specific antigens, like PSA, prostate specific membrane antigen (PSMA), or prostatic acid phosphatase (PAP), are targets of several antitumour vaccines.
Antigen-loaded DC, as well as synthetic peptides, manipulated tumour cells, and viral vectors, are some vaccination strategies used to improve the prognosis and quality of life of patients with advanced or recurrent PCa.33
Sipuleucel-T
Sipuleucel-T (Provenge®) is an autologous cell-based immunotherapy for the treatment of asymptomatic or minimally symptomatic mCRPC and it remains the only FDA-approved vaccine for PCa.34 This vaccine activates an antitumour immune response against PAP, a secreted glycoprotein synthesised in prostate epithelium, usually increased during cancer progression.35 This personalised immunotherapy is the result of immune cells harvested from the patient via leukapheresis and incubated with a recombinant fusion protein (PA2024) consisting of PAP and granulocyte-macrophage colony-stimulating factor (GM-CSF), to stimulate antigen-presenting cells and to obtain mature DC. Sipuleucel-T is then reinfused into the patient to evoke an antitumour immune response against PCa cells expressing PAP. After three cycles over 4 weeks, patients develop an appreciable specific T cell activation and production of antibodies against the fusion protein.36 Three randomised Phase III clinical trials were completed and all showed a delay in disease related pain.7,17,18 Sipuleucel-T is generally well tolerated, with only few adverse events, such as chills, fever, and headache, due to the cytokine release induced by vaccine. The time of disease progression was not modified with statistical significance, but an increase of overall survival (OS) (25.9 months versus 21.4, and 19.0 months versus 15.7, respectively) was reported in two studies that analysed Sipuleucel-T versus placebo in asymptomatic mCRPC patients.37,38 Results from the IMPACT study,37 which enrolled asymptomatic, symptomatic, or minimally symptomatic mCRPC patients, highlighted a 4.1 month improvement in the median OS and no significant difference in time to disease progression (14.6 weeks versus 14.4 weeks).39 A decline in PSA was reported in <10% patients.
Several studies were focussed on further understanding the immune pathway induced by Sipuleucel-T, as well as on identifying biomarkers that better correlate with clinical outcomes for therapies like Sipuleucel-T.
An open-label Phase II neoadjuvant trial40 showed that Sipuleucel-T induced infiltration of T cells into the tumour and recruitment of activated effector T cells (CD4+ and CD8+) into the PCa microenvironment, an increase of T cells with an activated phenotype at the tumour-stroma interface, and a systemic antigen specific T cell response.41
Additionally, Sipuleucel-T elicits IgG production against PAP and non-target tumour antigens, which are believed to contribute to the clinical efficacy of this vaccine.42 Moreover, the improvement of OS induced by Sipuleucel-T may be correlated to the long-lasting antigen-specific cytotoxic T cells and to the resulting tumour cell death.43 The promotion of a Th1 immune response was associated with a PSA decline too.44
Sipuleucel-T, like other immunotherapies, seems to be mostly effective in patients with a low tumour burden and a less compromised immune system.13
DCVAC/PCa
DCVAC/PCa represents another type of DC-based vaccine and consists of autologous poly I:C-activated DC loaded with a PSA-positive PCa human cell line, LNCaP, ultraviolet irradiated for safety.45 Multiple Phase I/II clinical trials have explored the effects of the combination of this vaccine with docetaxel or with radiotherapy, as discussed later.45,46
Blood dendritic cell antigens/1 BDC-01
Blood dendritic cell antigen (BDCA)/1 BDC-01 is an autologous vaccine prepared by pulsing autologous CD1+ cells from leukapheresis with a cocktail of HLA-A*0201-restricted peptides (PSA, PAP, PSMA, and control influenza peptide) mixed with keyhole limpet haemocyanin. The vaccine has been shown to be well tolerated, but no modification of disease was noted and PSA levels remained unchanged.47
PROSTVAC-VF
PROSTVAC-VF (PSA-TRICOM) uses two different recombinant pox-virus vectors encoding transgenes for TRICOM, containing a triad of T cell costimulatory molecules (LFA-3, B7.1, and ICAM-1), and PSA as primary immunotherapy, followed by boosters employing fowlpox. The vaccinia virus acts as an immunogenic vector; the infected cells undergo necrosis, releasing PSA that is subsequently processed by immature DC. Cell necrosis also releases pro-inflammatory signals that can further activate more DC.48 These mechanisms induce T cell-specific immune response against PSA with an increase in median survival of 8–9 months and a reduced mortality rate.49 A large international Phase III trial of asymptomatic or minimally symptomatic mCRPC treated with PROSTVAC-VF combined with GM-CSF was halted prematurely because no OS benefit was seen.50
A randomised Phase II placebo-controlled, double-blind trial of PSA-TRICOM was conducted to analyse its immunologic and clinical effects in localised PCa;17 it was well-tolerated and induced a significant increase in CD4+ and CD8+ cells.51
GVAX
GVAX (i.e., GM-CSF tumour cell vaccine) uses two PCa cell lines, LNCaP (androgen-sensitive derived from a lymph node metastasis) and PC3 (androgen-insensitive derived from bone metastasis), as the antigen source, genetically modified to express GM-CSF and then irradiated for safety.52
Two Phase III trials were performed, which showed good toleration and an increase of immunogenic response, parallel to an increase of OS rates; however, both trials were discontinued early because of the low probability to meet their primary endpoint, indicated in duration of survival.13,52
Listeria monocytogenes vaccine
Listeria monocytogenes (Lm) vaccine uses Listeria, an intracellular pathogenic bacterium that directly targets and activates DC, inducing both innate and adaptive immune responses against encoded heterologous antigens.53 Two modified Lm, lacking their pathogenic features but maintaining their immunogenicity, have been developed for PCa. One, ADXS31-142, a vaccine with a live attenuated Lm-listeriolysin O (LLO) targeting PSA, was constructed to secrete an antigen-adjuvant fusion protein, consisting of a truncated fragment of the LLO fused to PSA. Lm-LLO/PSA caused regression of established PSA expressing tumours and induced specific cellular immune responses in mice, represented by a lower number of tumour infiltrating Tregs and T cells specific for PSA that recognise and lyse PSA-peptide pulsed target tumour cells.54 ADXS31-142 is ongoing in mCRPC patients. Phase I/II, open-label, multicentre, non-randomised trials22 are evaluating the safety and tolerability of ADXS31-142 alone and in combination with pembrolizumab, a programmed death receptor 1 (PD-1) inhibitor.54
The other Listeria vaccine, ADU-741, live attenuated double deleted, involves a strain with the deletion of two virulence genes, actA and internalin B, and it is engineered with several prostate-associated antigens.55 Currently, a Phase I clinical trial has investigated the optimum dose of ADU-741 to induce immunogenicity in patients with mCRPC.56 Vaccination therapies are summarised in Figure 1.
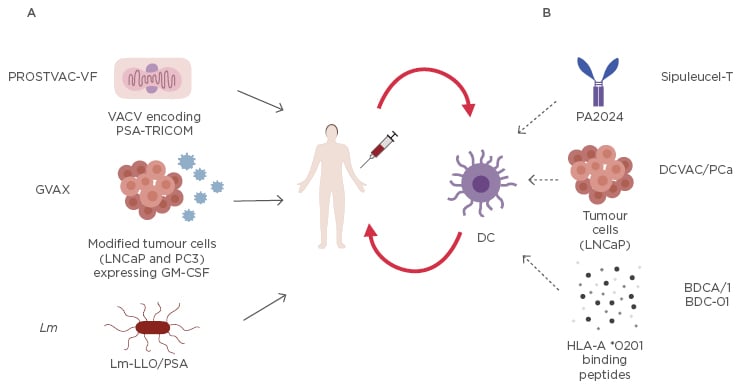
Figure 1: Current mentioned vaccination strategies for prostate cancer.
A) These compounds are directly injected into the patient: vaccinia (primary immunotherapy) and fowlpox (employed for 6 boosters), both engineered with recombinant poxvirus that express PSA and 3 costimulatory molecules named TRICOM (LFA-3, B7.1, and ICAM-1), are used in PROSTVAC-VF; two modified ultraviolet-killed tumour cell lines, androgen sensitive derived from a lymph node metastasis, and androgen insensitive derived from bone metastasis (PC3), express GM-CSF in GVAX; recombinant live attenuated Lm that expresses and secretes PSA fused to a non-functional LLO in Lm-LLO/PSA.
B) Dendritic cells are first harvested via leukapheresis from the patient, then activated ex vivo, expanded, and reinfused in the same patient. Dendritic cells are activated with: recombinant fusion protein (PA2024) consisting of PAP and GM-CSF in Sipuleucel-T; LNCaP cells incorporated with Poly I:C in DCVAC/PCa; or HLA-A*0201 binding peptides PSA, PAP, PSMA, and control influenza peptide mixed with keyhole limpet haemocyanin in BDCA/1 BDC-01. The activated dendritic cells are expanded and reinfused into the same patient.
GM-CSF: granulocyte-macrophage colony-stimulating factor; Lm: Listeria monocitogenes; Lm-LLO: Listeria monocitogenes -listeriolysin O; LNCaP: lymph node metastasis; PAP: prostatic acid phosphatase; PCa: prostate cancer; Poly I:C: polyinosinic-polycytidylic acid; PSA: prostate specific antigen; PSMA: prostate specific membrane antigen.
IMMUNE CHECKPOINT INHIBITORS
Tumour cells can also escape from the immune system through pathways involving immune checkpoints; these recognise surface-expressed ligands on self-tissues and dampen unwanted immune activation maintaining homeostasis and self-tolerance.57 Several immune checkpoint inhibitors (ICI) that target such molecules expressed on immune or tumour cells are under evaluation as to whether they enhance the antitumour immune response.
CTLA-4, expressed on activated T cells and Tregs, downregulates the extent of T cell activation by determining the balance with CD28 signals. CTLA-4 binds the ligands B7-1 (CD80) and B7-2 (CD86) with a higher affinity than CD28.58 The binding to CTLA-4 limits T cell expansion.59
IPILIMUMAB
Ipilimumab, a fully humanised monoclonal antibody that binds and blocks CTLA-4, restoring an anti-tumour immune response, was the first molecule FDA-approved in 2011 for advanced melanoma. Currently, many clinical trials with this drug are ongoing on several different types of tumours, including PCa tumours.
Two Phase III studies analysing the effects of combining ipilimumab with a low dose of radiotherapy in chemotherapy-naïve asymptomatic or minimally symptomatic mCRPC, showed that ipilimumab had no benefit in OS, but slightly improved PFS, suggesting a possible antitumour activity.60 PD-1, a transmembrane glycoprotein predominantly expressed on T cells, interacts with PD-L1 and PD-L2 expressed on antigen-presenting cells, promoting tolerance and preventing tissue damage during chronic inflammation.
The interaction of PD-1/PD-L1 is the principal mediator of immune suppression and represents one of pivotal mechanism of immune escape by tumours.61 PD-1/PD-L1 blockade could restore immune functions, resulting in a reduction of tumour mass and metastatic spread. Generally, PCa shows upregulation of PD-L1 expression as an adaptive response to changes in the immune microenvironment and PD-L1 is expressed by primary PCa and is increased in mCRPC.62 This is the rationale of targeting PD-1/PD-L1 for the treatment of mCRPC.
Nivolumab
Nivolumab is a human IgG4 anti PD-1 and is FDA-approved for metastatic melanoma and for other tumours. No objective responses were reported in PCa treated with nivolumab. Indeed, only 1 of 17 patients with CRPC enrolled in a nivolumab trial showed a 28% reduction in measurable lesions.58
Pembrolizumab
Pembrolizumab is a humanised antibody targeting PD-1 and is FDA-approved for metastatic melanoma and recently for any unresectable or metastatic solid tumour with certain genetic anomalies (mismatch repair deficiency or microsatellite instability). It has shown a favourable side effect profile in mCRPC.63 The clinical benefits are under evaluation in monotherapy and in combination therapy.25
COMBINED THERAPY
Combination regimens have been proposed to optimise tumour immunogenicity and immune response in PCa. Several trials are evaluating the combination of different immune response modulators and the combination of immunotherapy with conventional therapies to improve cancer management.
For example, a combined therapy using different compounds targeting immune checkpoint blockade has been applied to PCa patients harbouring mutations in DNA-repair genes and shown encouraging clinical results.13 Mateo et al.48 have demonstrated that DNA repair defects (DRD) are detected in mCRPC patients during cancer progression: in 50 previously treated mCRPC patients, 16 patients associated with DNA repair defects in tumour cells showed a response to olaparib. Therefore, PARP inhibitors (PARPi), such as olaparib, could be a promising therapy.64 PARPi induce cell death and lead to an increased mutational load in cancer cells. In vitro studies and mouse models have shown that PARPi enhance IFN and TIL, decrease myeloid-derived suppressor cells, and also induce PDL-1.65 These data support the rationale for combining PARPi and ICI. A radiographic and/or PSA positive response was reported in patients treated with durvalumab, a human Ig blocking PD-L1, and olaparib.28,66
Moreover, combined ipilimumab and nivolumab was also evaluated in AR-V7-expressing mCRPC, an aggressive phenotype with poor PFS and OS, characterised by the expression of a mutated AR. Preliminary evidence suggested that AR-V7-positive tumours may be enriched for DRD, perhaps rendering them more sensitive to ICI. Ipilimumab plus nivolumab demonstrated encouraging efficacy in 40% of AR-V7-positive PCa with DRD mutations.24,67
Durvalumab with or without tremelimumab, a fully human monoclonal antibody anti-CTLA-4, is in Phase II study29 with mCRPC patients, addressed to evaluate safety, tolerability, and interference with the growth and spread of the tumour.
ADT can synergise with ICI. Indeed, it is reported that ADT initially elicits a short-lived antitumour Th1-type response, but subsequently it can stimulate immunosuppressive lymphocyte subsets. MDV3100 induces an increase of natural killer cells and a decrease in myeloid-derived suppressor cells. On the other hand, MDV3100 upregulates PD-1/PD-L1 in resistant MDV3100 tumour cells and DC giving the premise for the pembrolizumab efficacy.68 Pembrolizumab was added to MDV3100 in patients with progression and this Phase II trial reported a durable decline of PSA in a subset of patients and a radiographic positive response.26,69
The efficacy and safety of atezolizumab, another anti PD-L1, is under investigation in a Phase III randomised, multicentre trial, combined with MDV3100 and also compared with MDV 3100 alone in mCRPC patients who progressed after abiraterone acetate.30
Another combining therapy concerns vaccines with ICI. A murine model showed that the increase of immune response was counteracted by expression of PD-1 on antigen-specific CD8+ T cells elicited with immunisation. However, the blockade of PD-1/PD-L1 during T cell activation with immunisation led to superior antitumour efficacy.70 Furthermore, patients previously immunised with either Sipuleucel-T or with a cancer vaccine containing plasmid DNA encoding human PAP (pTVG-HP) developed PD-1-regulated immune responses. Additionally, after vaccination, circulating tumour cells showed increased expression of PD-L1.71
Moreover, Th1 promotion induced by Sipuleucel-T correlated with a PSA decline while a PSA progression correlated with the upregulation of genes associated to CTLA-4, again suggesting the possible role of these checkpoints in dampening treatment-induced immune responses.44 Based on this rationale, a clinical trial combining Sipuleucel-T with ipilimumab is ongoing for mCRPC.14
When combined therapies are chosen, identifying the best sequence is important. Recently, in a Phase II trial, identified as STAND,72 patients with biochemically recurrent PCa were treated with Sipuleucel-T and ADT. When Sipuleucel-T was administered prior to ADT, a superior cellular immune response was induced, about 2-fold higher than the reverse sequence.
The cancer vaccine pTVG-HP combined with pembrolizumab was evaluated in mCRPC patients and its efficacy was augmented by concurrent PD-1 blockade. The development of PAP-specific Th1 and CD8+ T cell infiltration of the tumour was associated with a PSA decline, leading to a shrinkage of the tumour in some patients. More results are expected from the indicated study.73
The DCVAC/PCa vaccine in patients with mCRPC that have already received docetaxel induced an immune modulation, as indicated by the increase of antigen-specific T cell against PSA, detected after the fourth dose, and a significant decrease in the frequency of Tregs. Following 12 doses, the median PSA doubling time (PSADT) had increased from 5.67 prior to immunotherapy to 18.85 months.46,47 Other studies including more patients and longer times of follow up are needed.16
PROSTVAC-VF combined with escalating doses of ipilimumab in 30 patients with mCRPC resulted in a decline in PSA in 58.0% of patients, and 73.3% of patients remained alive after 24 months versus 53% of patients treated with PROSTVAC-VF alone. Approximately 20% of patients treated with PROSTVAC-VF plus ipilimumab at higher doses remained alive at 80 months.49 A Phase II study focussed on immune response18 and a Phase I/II study of PROSTVAC-VF added to nivolumab with or without ipilimumab, with safety and changes in T cell infiltration as the primary endpoints, is ongoing.19 Furthermore, PROSTVAC-VF plus docetaxel20 or MDV310021 are under evaluation.
A trial combining LmLLO/PSA with pembrolizumab is also ongoing, the first results of which show that this combination appears safe and tolerable, and showed promising activity compared to monotherapy. More analyses are underway in this trial.22
Recent data show that radium-223 induces T cell-mediated lysis in different tumour types, including PCa tumours;74 therefore, combining this drug with immunotherapies may have additional clinical benefit. Radium-223 combined with pembrolizumab,27 atezolizumab,31 or Sipuleucel-T15 is under evaluation in Phase I and Phase II studies.4 Similarly, radiotherapy is currently undergoing Phase III study combined with ProstAtak (AdV-tk + valacyclovir), a viral cancer vaccine.23
ADOPTIVE T CELL THERAPY
Adoptive T cell therapy is another strategy for achieving an effective immune response against tumours. In this therapy, T cells from patients are genetically engineered and then reinfused. The development of chimeric antigen receptors (CAR) permits the targeting of tumour antigens with lower restrictions, such as TIL or lymphocytes genetically engineered to express a relevant T cell receptor. In CAR-T therapy, the specificity of antibodies is associated with cytotoxic activity of T cells to target tumour cells. Autologous T cells from patient are engineered ex vivo to express a CAR directed against TAA and are then reinfused back to the patient, to recognise and destroy tumour cells.
However, CAR-T can also induce many adverse effects that restrict the efficacy of such therapies.75
Until now, CAR-T targeting CD19 cells have been reported in studies to be a success in the treatment of haematological malignancies, and tisagenlecleucel was FDA-approved in 2017 for children and young adults with leukaemia. The application of this strategy to solid tumours is still being developed.
In a PCa mouse model, CAR-T cells constructed with anti-PSMA and CD28 as costimulatory molecules induced a near complete disappearance of the tumour in 3 weeks.76 A recent Phase I trial,32 using PSMA CAR-T cells, induced a reduction of PSA in two of five treated patients, and it was speculated that the lowering of plasma IL-2 interfered negatively.77
CONCLUSION
Until now, immunotherapy for PCa has shown less efficacy compared to in some other tumours.58,78 Low mutational burden and a suppressive tumour microenvironment may negatively affect immunotherapy in PCa. Combinations of therapies, either involving multiple immunotherapies and/or standard chemotherapy, radiotherapy, and hormone therapy, might improve treatment outcomes and are under evaluation. It will also be necessary to determine the strategies to optimise timing and sequencing of combination therapy for maximal efficacy.