Abstract
Overactive bladder syndrome (OAB) can be effectively treated with onabotulinumtoxinA, which is recommended as a third-line therapy. Despite its proven effectiveness and safety, a large number of patients, who may benefit, are not receiving onabotulinumtoxinA for refractory OAB. One reason is patient and/or physician concerns and misunderstandings regarding the requirement for clean intermittent catheterisation (CIC) with post-void residual (PVR). At present, there is no published consensus on the management of patients with incomplete bladder emptying after onabotulinumtoxinA treatment. There is thus a need for guidance on management of elevated PVR associated with onabotulinumtoxinA due to misunderstandings and great deal of confusion regarding the effects of onabotulinumtoxinA on bladder emptying. Incomplete bladder emptying following onabotulinumtoxinA treatment has been frequently referred to as acute urinary retention (AUR), but this is not an accurate description for this adverse event. The majority of patients with incomplete emptying post-onabotulinumtoxinA treatment can spontaneously void, so true AUR is rare. In both prospective and retrospective studies, variable definitions and different management approaches have led to a wide range in the reported incidence of PVR ≥200 mL, CIC and AUR of 0.4–41.4%, 1.0–30.0%, and 2.0–35.0%, respectively. This expert consensus provides important guidance on the appropriate management of incomplete voiding of the bladder post-onabotulinumtoxinA injection and the recommendations for when to implement CIC.
INTRODUCTION
Overactive bladder syndrome (OAB) has a prevalence of 12–36%.1 Anticholinergics have been the mainstay of pharmacological treatment for many years.2 However, adverse events (AE) such as dry mouth, constipation, dry eyes, dizziness, or cognitive issues (e.g., dementia), may limit their use.2,3 The β3-adrenoceptor agonist mirabegron is an alternative oral therapy, but is contraindicated in patients with severe uncontrolled hypertension4,5 and is associated with headache, urinary tract infection (UTI), tachycardia, and increased blood pressure.6 Many patients are also non-compliant and therefore do not achieve the optimal benefits of oral medication.6
OnabotulinumtoxinA (BOTOX®, Allergan, Dublin, Ireland) is approved for the treatment of OAB with symptoms of urgency urinary incontinence, urgency, and frequency, in adults who have an inadequate response to, or are intolerant of, an anticholinergic medication.7 The efficacy and safety of onabotulinumtoxinA in the treatment of OAB have been demonstrated in numerous clinical studies and routine clinical practice.8-19 OnabotulinumtoxinA improves patients’ quality of life (QoL)13,20-25 with more patients experiencing complete resolution of urinary incontinence, longer duration of effects versus oral medications,13,24,26 and is a cost-effective treatment option.26-28 OnabotulinumtoxinA is recommended as third-line therapy for OAB in the 2019 European Association of Urology (EAU) Guidelines on Urinary Incontinence29 and the 2015 American Urological Association (AUA)/Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU) guidelines.30
Despite the proven effectiveness of onabotulinumtoxinA, a large number of patients who may benefit are not receiving onabotulinumtoxinA for refractory OAB. One reason is patient and/or physician concerns regarding AE, particularly relating to incomplete bladder emptying leading to a raised post-void residual (PVR). In some cases, a raised PVR requires treatment with clean intermittent catheterisation (CIC).
There is no published consensus on management of incomplete bladder emptying after onabotulinumtoxinA treatment. Protecting the upper urinary tract, bladder, and controlling UTI are typically considered the clinical reasons for treating incomplete bladder emptying. Therefore, there is a need for guidance on the management of elevated PVR associated with onabotulinumtoxinA treatment. The authors review the definitions of AE and reported PVR/CIC rates with onabotulinumtoxinA and provide guidance on the management of elevated PVR and initiation of CIC.
DEFINITION OF POST-VOID PRESIDUAL, CLEAN INTERMITTENT CATHERISATION, AND ACUTE URINARY RETENTION
There has been a great deal of confusion regarding the effects of onabotulinumtoxinA on bladder emptying. Incomplete bladder emptying following onabotulinumtoxinA treatment has been frequently referred to as acute urinary retention (AUR),9,15-18 which may require treatment with CIC. However, the term AUR in this context is not an accurate description for this AE because AUR suggests that the bladder is unable to void at all, when, in fact, the majority of patients with incomplete emptying post-onabotulinumtoxinA treatment can spontaneously void. True AUR is rare and understanding this is important to provide an accurate description to patients of this possible AE.
According to ICS terminology, PVR is defined as the ‘volume of urine left in the bladder at the completion of voiding.’31,32 However, there is no definition of what constitutes a clinically significant value for an elevated PVR or how many measurements should be taken. CIC (also known as CISC: clean intermittent self-catheterisation) is defined as ‘use of a clean technique. This implies ordinary hand and genitals washing techniques and use of disposable or cleansed reusable catheters.’32,33 Crucially, it also requires intermittent insertion and immediate removal (as opposed to an indwelling catheter) and use over a prolonged period of time. AUR is defined as a ‘complaint of a rapid onset, usually painful suprapubic sensation (from a full bladder) due to the inability to void (non-episodic), despite persistent intensive effort.’31,32
A patient with incomplete bladder emptying post-onabotulinumtoxinA will develop a raised PVR. If this PVR is significantly raised, the patient may require CIC to aid bladder emptying until sufficient bladder voiding function returns. The majority of these patients will not have AUR as they are able to spontaneously void. Therefore, the use of CIC with onabotulinumtoxinA is not synonymous with AUR.
In the Phase III EMBARK onabotulinumtoxinA studies,34,35 protocol specific AE definitions used terminology based on MedDRA coding (i.e., urinary retention and residual urine volume), and this terminology is not consistent with ICS terminology and definitions. These trials were designed to allow for some investigator discretion to manage AE, to ensure a consistent approach across sites, but more importantly to ensure patient safety in the first large scale pivotal trial. In addition, the trials were conducted for regulators to gain marketing approval for onabotulinumtoxinA. Patients were required to commence CIC when they met certain criteria (see below) that may not be reflective of real-world practice. In addition, if a patient started CIC, this was classed as an AE of urinary retention (even if they could still spontaneously void). Based on this criterion, the prescribing information for onabotulinumtoxinA indicates a urinary retention rate of 6.5%.7 When comparing the definition of urinary retention from the pivotal trials to that generally accepted among physicians based on ICS definitions, it is not surprising that lower rates of AUR and CIC are reported in real-life practice.36 Patients often have a misperception regarding CIC and are frequently apprehensive because they assume this is the same as having an indwelling catheter. Patients also often have a misperception that incomplete emptying (particularly when described as urinary retention) is an intolerable and painful condition.
These misunderstandings about CIC and AUR may lead to patients being incorrectly counselled about the potential need for CIC or what incomplete bladder emptying entails and may therefore deter many patients from considering treatment with onabotulinumtoxinA.
POST-VOID RESIDUAL, CLEAN INTERMITTENT CATHETERISATION, AND ACUTE URINARY RETENTION RATES WUTG ONABOTULINUMTOXINA 100 U
In both prospective and retrospective studies, the variable definitions of PVR, CIC, and AUR, and different management approaches have led to a wide range in incidences and durations of CIC and AUR.
EMBARK Phase III REGISTRATIONAL STUDIES
The two EMBARK studies34,35 were the pivotal trials that led to the global registration of onabotulinumtoxinA 100U.9,15,17 CIC was initiated if the PVR was 200–<350 mL with associated symptoms which in the opinion of the investigator required CIC or if the PVR was ≥350 mL irrespective of symptoms. An AE of urinary retention should have been reported for all patients requiring CIC. The criteria for CIC cessation was dependent on patient symptoms, a PVR <200 mL, and could have been affected by the timing of study visits when PVR could be reassessed.
In EMBARK 1,34 the proportion of patients who initiated CIC during treatment cycle 1 was 6.1% (17/278) with onabotulinumtoxinA versus none with placebo.15 Of 17 patients who required CIC, 10 (58.8%) had a duration of CIC of ≤6 weeks. A total of 21 patients (7.6%) had a PVR 200–<350 mL; however, only 6 (2.2%) patients had a PVR 200–<350 mL requiring initiation of CIC. Thus, 15 (71.5%) patients with a PVR 200–<350 mL were deemed by the investigator not to have symptoms requiring CIC. Of the total, 10 (3.6%) patients had a PVR ≥350 mL requiring CIC per protocol.
In EMBARK 2,35 the proportion of patients who initiated CIC during treatment cycle 1 was 6.9% (19/274) with onabotulinumtoxinA versus 0.7% (2/270) with placebo.9 In those who performed CIC, it was started within the first 12 weeks following treatment in almost all patients. A total of 17 patients (6.2%) had a PVR 200–<350 mL; however, only 7 (2.6%) patients had a PVR 200–<350 mL required initiation of CIC. Thus, 10 (58.8%) patients with a PVR 200–<350 mL were deemed by the investigator not to have symptoms requiring CIC. Eleven patients (4.0%) had a PVR of greater than or equal to 350mL, of which 10 patients were commenced on CIC.
Patients who completed the EMBARK 1 and 2 studies could enter a 3-year extension study and of those who entered the proportion of patients initiating de novo CIC was calculated for each treatment cycle.16 The proportion of patients who required de novo CIC was 4.0%, 1.7%, 1.4%, 1.6%, 0.6%, and 0.8% in treatment cycles 1–6, respectively (the first cycle occurring in EMBARK 1 or 2). The median duration of CIC was 3.1–8.3 weeks.
A post hoc pooled analysis of the two EMBARK studies that assessed the rates of CIC in different age groups showed the risk of CIC was low in all age groups. CIC rates were lowest in the <40-year-old group (1.1%) and increased with age in the 40–49, 50–59, 60–69, and ≥70-year-old groups, (3.2%, 5.3%, 5.3%, and 7.2%, respectively).26 The mean duration of CIC in the <40 and 40–49-year-old groups was 3 and 44 days and ranged from 78 to 88 days in the other age groups.
A recent analysis of pooled data (N=551) from both EMBARK studies of patients treated with onabotulinumtoxinA showed that the mean PVR post treatment was highest at the 2-week timepoint. Of the 551 patients, 493 (89%) had a PVR <200 mL, 38 (6.8%) a PVR 200–350 mL, and only 20 (3.6%) a PVR >350 mL (Figures 1 and 2) (data on file).
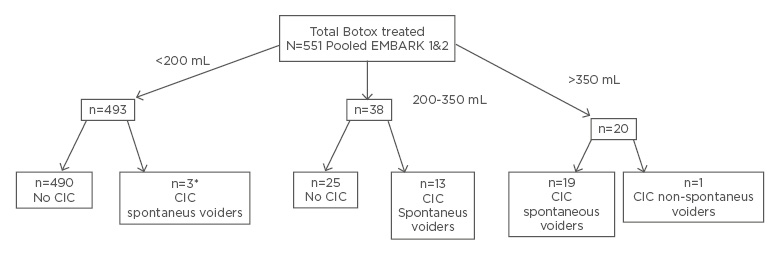
Figure 1: Disposition of onabotulinumtoxinA-treated patients in the pooled EMBARK population with regards to maximum post treatment PVR in the 12 weeks after treatment.
*CIC cases were deviation from the protocol.
CIC: clean intermittent catheterisation.
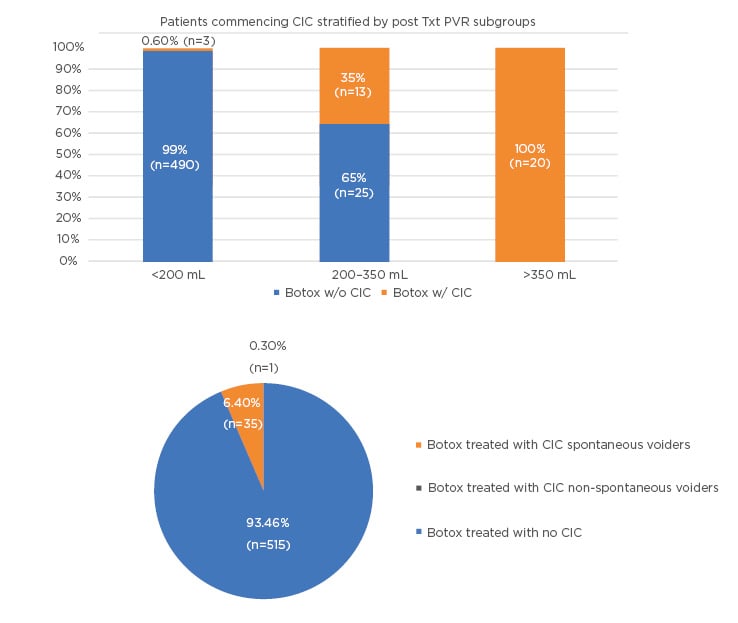
Figure 2: (A) Patients initiating CIC by max PVR subgroups. (B) CIC patients spontaneous versus non-spontaneous in the pooled EMBARK population.
CIC: clean intermittent catheterisation; PVR: post-void residual.
Only 1 patient in the >350 mL group (0.2% of the overall population) had true AUR. A post hoc pooled analysis of three Phase III studies9,15,24 and a Phase IV study37 evaluated the risk of CIC following retreatment with onabotulinumtoxinA 100 U in 831 patients.38
The cumulative incidence of CIC after the second treatment was 5.5% (26/469 patients); 9 patients (1.9%) required CIC in both treatment cycles and 17 patients (3.6%) only in treatment cycle 2. There was no increased risk of CIC with onabotulinumtoxinA retreatment and only 9 patients (1.9%) with CIC after the initial treatment required CIC again following retreatment.
Prospective Clinical Studies
In other prospective clinical studies, the reported rates of PVR ≥200 mL, CIC and AUR were 0.4–10.0%, 1.0–10.0%, and 4.3–9.1%, respectively, with onabotulinumtoxinA 100 U (Table 1).13,24,39-42 Differences in the rates of CIC is partly due to the variation in the requirement for CIC (Table 1). These clinical studies confirm the lack of consensus on what criteria constitutes a requirement for CIC.
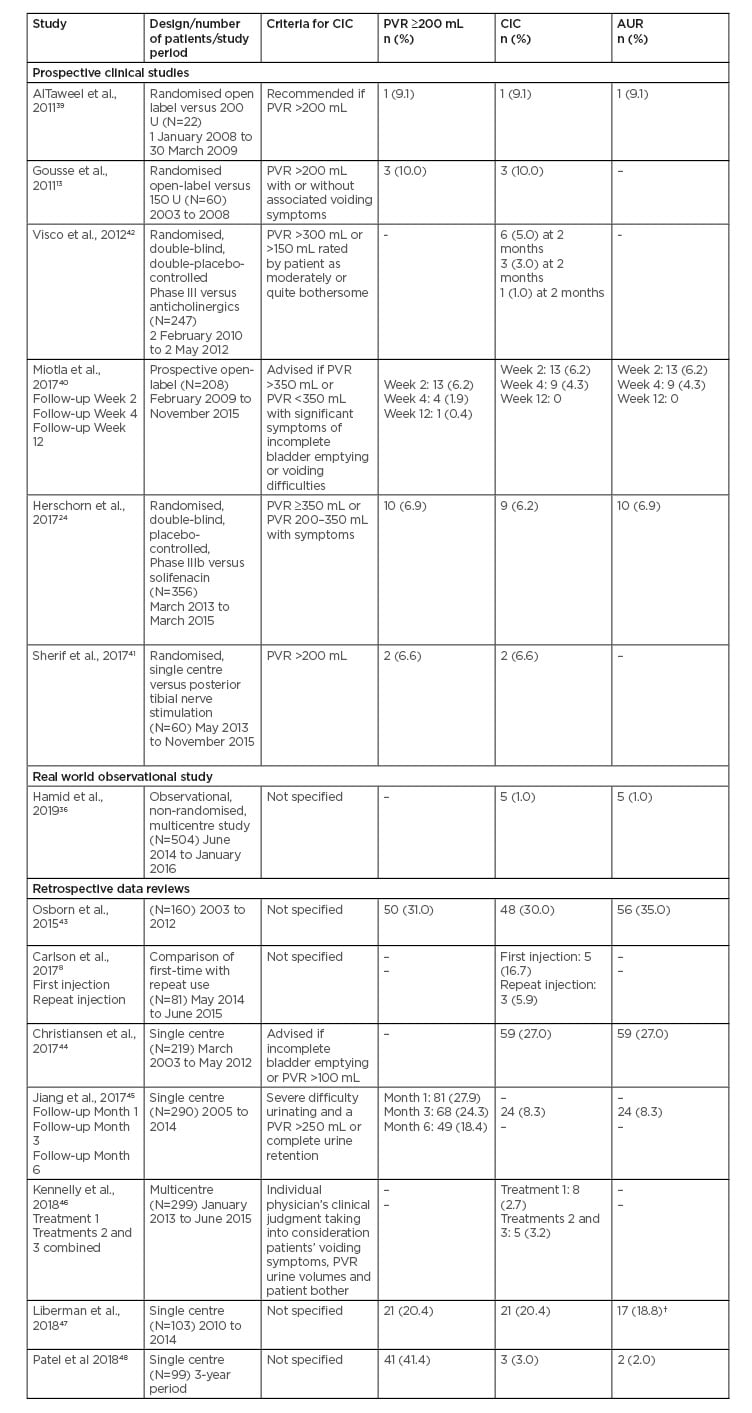
Table 1: Rates of post-void residual, clean intermittent catheterisation, and acute urinary retention with onabotulinumtoxinA 100 U in other studies and retrospective reviews.
AUR: acute urinary retention; CIC: clean intermittent catheterisation; OAB: overactive bladder; PVR: post-void residual; UUI: urgency urinary incontinence.
†17 of 90 patients who received the 100 U dose.
Therefore, the rates of CIC and urinary retention in these trials may not relate to real-world clinical practice.
Real World Observational Study
In a prospective observational study (N=504) of real-world use of onabotulinumtoxinA for the management of OAB (GRACE), the criteria for urinary retention and CIC were not defined and left to the discretion of the physician. There was a very low rate of urinary retention/CIC (1.0%), suggesting that in clinical practice, urinary retention with onabotulinumtoxinA is much less frequent than in clinical trials.4
Retrospective Data Reviews
In retrospective data reviews of onabotulinumtoxinA 100 U, the reported rates of PVR >200 mL, CIC and AUR were 20.3–41.4%, 3.0–30.0%, and 2.0–35.0%, respectively (Table 1).8,43-48
A private practice study of 103 patients undergoing their first treatment with onabotulinumtoxinA reported a high CIC rate of 20.4%.47
However, 13% of patients received a onabotulinumtoxinA dose of 200 U rather than the approved 100 U dose. An editorial comment on this study states that great care should be taken when comparing this and other studies assessing the risk of the need for CIC as the reported rates vary widely.49 The comment notes that the definition of AUR could be responsible for the higher rate.
In a 10-year data review, post-procedure urinary retention was defined as any patient who was started on daily CIC or had an indwelling catheter fitted.43
The high rates of CIC (30.0%) and AUR (35.0%) were primarily due to the low threshold for initiation of CIC and the inclusion of patients with a pre-treatment PVR >100 mL. They could also be due to the fact that some patients received a higher onabotulinumtoxinA dose of 200 U, but the number of patients was not specified. In addition, 36 patients (23.0%) had diabetes, of whom 16 (44.4%) had retention. Patients with diabetes were twice as likely to develop urinary retention compared with non-diabetic patients.
In a single-centre review in Taiwan of 290 patients, AUR was defined as severe difficulty urinating and a PVR >250 mL or complete urine retention.45 Patients who developed AUR were instructed to perform CIC. AUR occurred in 8.3% of the patients. In a multicentre review of multiple treatments, the CIC rate was 2.7% after treatment 1 and 3.2% after treatments 2 and 3 combined.46 In a review of 99 female patients, which defined AUR as an inability to void requiring CIC and the presence of symptomatic incomplete bladder emptying, defined as symptoms of poor emptying (i.e., straining, weak stream, or the sensation of incomplete emptying) with a PVR ≥350 mL, only 3 patients (3.0%) required CIC.48
Retrospective reviews have a number of caveats, including the quality of the data, which must be considered when assessing the information; however, the above studies illustrate the lack of consistency in real-world practice in treating onabotulinumtoxinA-related cases of incomplete voiding of the bladder.
ASSESSING THE NEED FOR CLEAN INTERMITTENT CATHETERISATION
There is no consensus on when to initiate CIC. Routine administration of CIC based on an arbitrary PVR volume is unlikely to be beneficial for patients. Protecting the upper urinary tract, protecting the bladder from over-distension injury, and controlling UTI are the chief concerns for clinicians when deciding when an increased PVR represents a danger to the patient. It is important to recognise that incomplete bladder emptying after onabotulinumtoxinA injection is associated with decreased detrusor contractility and, therefore, the risk of reflux is highly unlikely. In the authors’ opinion, there is no need for patients to practice CIC before receiving treatment with onabotulinumtoxinA because the likelihood of requiring CIC is very low (1.0–6.5%). Practicing CIC unnecessarily causes concern for patients and an increased risk of UTI. Pre-emptive training may only be needed in rare cases, e.g., in someone with impaired neurological function where it is not clear whether or not they will be able to perform CIC. Factors such as the injection paradigm, patient age, baseline PVR, and other risk factors, such as men with benign prostatic hyperplasia, should be considered when assessing the risk for CIC. The principle of voiding efficiency (VE) may be helpful when deciding whether to use CIC. Incomplete emptying of the bladder can be determined by the VE, which is defined as the volume voided divided by the total bladder volume (voided volume + post void residual) x 100. CIC should be considered if the VE is ≤40%. If the VE is between 40% and 60%, the patient should be followed regularly, and CIC started if voiding symptoms indicate.
CONSENSUS STATEMENT ON APPROPRIATW MANAGEMENT OF ELEVATED POST-VOID RESIDUAL POST-VOID RESIDUAL POST-ONABOTULINUMTOXINA INJECTION
Based on a consensus discussion and review of the literature, the authors propose the
following recommendations.
Monitoring Voiding Efficiency / Post-Void Residual
PVR should be assessed around 2 weeks following treatment (or sooner if the patient has early symptoms of voiding dysfunction). VE should also be assessed (to assess voided volume, a frequency volume chart or office voided volume can be used). If the VE is ≤40%, further lower urinary tract symptom assessment should be evaluated prior to CIC initiation.
PVR should be reviewed at regular follow-up intervals if the PVR is raised and the VE is ≤40%. The frequency of assessment should be based on the patient’s symptoms and the magnitude of the PVR and VE.
Initiation of Clean Intermittent Catheterisation
The decision to initiate CIC should be based most importantly on bothersome symptoms and can be further supported by a VE <40% and a PVR >200mL.
Follow-Up/Repeat Post-Void Residual Assessment Once Clean Intermittent Catheterisation Has Been Initiated
The frequency of assessments will be dictated by clinical circumstances and will be more important in patients with a borderline raised PVR, as this is less relevant in a patient who is using CIC. For example, measurement should be conducted every 2–4 weeks in a borderline patient unless symptoms change.
Recommendations for Stopping Clean Intermittent Catheterisation
Patients who are using CIC should be advised that they can stop CIC if they are asymptomatic (no longer have bothersome symptoms) or if the VE is >40%.
Counselling Patients
In addition to explaining the full benefits and risks per the product label (SmPC), patients should be counselled by explaining:
The benefits of onabotulinumtoxinA versus other pharmacological therapies to significantly improve urgency urinary incontinence, with many patients becoming completely dry.
Patients may have some degree of incomplete emptying that usually is not bothersome or harmful and does not require intervention. It can easily be assessed in the office painlessly by ultrasound.
The real-world CIC rate should be explained, ideally supplemented with the rates in the physician’s own practice, if known.
CIC should be explained clearly (including that it is intermittent, discrete, performed by the patient and is temporary, with most patients managing with no issues). Ideally a CIC catheter should be shown to patients.
Patients should understand that onabotulinumtoxinA will need to be periodically repeated, as based on our experience, on average every 6 months (no more frequently than every 3 months per the package insert) and that each time the patient receives treatment there is a small risk of a raised PVR that may need CIC.
Patients often have a fear of urinary retention or catheterisation that is not understood by their doctor and therefore appropriate counselling is needed so the patient is not deterred from having appropriate treatment. Appropriate and accurate wording should be used to explain what may happen post-treatment. For example, “there is a risk that voiding may be inefficient, which may or may not be symptomatic. In most cases this does not need treatment but in some patients, CIC is an option to help manage the bladder until function returns to a sufficient level. When CIC is needed, it is usually only for a short duration of time.”
CONCLUSION
Incomplete bladder emptying following onabotulinumtoxinA treatment has been frequently referred to as urinary retention or AUR, but this is not an accurate description for this AE. The majority of patients with incomplete emptying post-onabotulinumtoxinA treatment can spontaneously void; therefore, true AUR is rare. This expert consensus provides important guidance on the appropriate management of incomplete voiding of the bladder post-onabotulinumtoxinA injection and the requirements for initiating and stopping CIC.








