Abstract
Objectives: To evaluate the efficacy of the ZSI 375 artificial urinary sphincter (AUS) (Zephyr Artificial Sphincter, Geneva, Switzerland) for the treatment of patients with post-radical prostatectomy urinary incontinence.
Methods: This was a retrospective, non-randomised, multicentre study open to patients with moderate-to-severe urinary incontinence due to intrinsic sphincter deficiency after radical prostatectomy. Efficacy and safety was evaluated on continence status, complications, and surgical revision.
Results: Twenty-seven patients were recruited between September 2013 and April 2016 and followed up to April 2017. Mean age was 67.70 years old (range: 55–78). Twenty-six patients (96.30%) presented with incontinence post-radical prostatectomy and one patient (3.70%) after post-radical prostatectomy plus adjuvant radiotherapy. Follow-up ranged from 12 months–42 months (mean: 27.11). The success rate was 88.90% after 12 months follow-up, 94.12% after 24 months follow-up and 83.33% after 36 months follow-up. Three patients (11.11%) presented with a scrotal infection, two patients (7.41%) suffered a urethral erosion, and two patients (7.41%) had a mechanical failure. The revision rate was 22.22% (six patients).
Conclusion: The follow-up time of this preliminary clinical study was long enough to demonstrate that the ZSI 375 AUS offers a good rate of continence to patients with urinary stress incontinence.
INTRODUCTION
The artificial urinary sphincter (AUS) AMS 800TM (American Medical Systems, Minnetonka, Minnesota, USA), first introduced in 1973, is regarded as the gold standard for the treatment of post-prostatectomy urinary incontinence (UI).1,2 American Medical Systems proposed five different versions of the device.3 The last version of the AUS, the AMS 800, was introduced in 1983 and it is still in use today. However, despite good long-term outcomes,4,5 the preparation and the procedure remain complex with a risk of complications such as erosion, infection, and mechanical failure ranging from 8–45%.6,7
Introduction of the antibiotic coating InhibiZone® had no significant impact on AUS infection or explantation rates.8 To ease AUS preparation and the procedure, the ZSI 375 AUS (Zephyr Artificial Sphincter, Geneva, Switzerland) has been developed (Figures 1 and 2). Manufactured mainly from medical grade silicone rubber, it is a one-piece device made up of two parts pre-connected by kink-resistant tubing. The adjustable cuff is a moulded curve and can be adjusted around the urethra. The pump and the pressure-regulating tank are grouped into the pump-unit and placed in the scrotum. It has no abdominal reservoir to reduce operating time and to avoid abdominal incision.9 This paper describes the results of our first experience with ZSI 375 device implanted in 27 male patients with stress UI because of an intrinsic sphincter deficiency (ISD) after radical prostatectomy.

Figure 1: Image of the ZSI 375 artificial sphincter.
Figure 2: Image of the AMS 800 and ZSI 375 artificial sphincters.
PATIENTS AND METHODS
This was a retrospective, non-randomised, multicentre study open to patients with moderate-to-severe UI due to ISD after radical prostatectomy. Moderate incontinence was defined as the use of three pads per day and severe incontinence as the use of four pads and more per day. The patient evaluation comprised a medical history, analysis of voiding (UI episodes, number of pads used per day), clinical examination, cystoscopy, and urodynamic exam to confirm ISD and to exclude bladder overactivity. The device implanted was the ZSI 375 AUS. It was performed through a perineal and inguinal incision as described by Staerman et al.9 Patients were discharged as soon as they could void spontaneously. The device was activated 8 weeks after the surgical procedure. Follow-up visits were planned 3, 6, and 12 months and then annually after the procedure. Patients had a clinical examination, urine analysis, bladder ultrasonography, and flow rate measurement.
Continence status was based on number of pads used per day with total continence (0 pads per day), social continence (0–1 pad per day) and improvement (2 pads per day and 50% fewer pads than at baseline). Success was defined as social continence plus improvement. All other continence statuses were considered as incontinence. Revision was defined as the device or one of its components needing replacement or temporary removal. During the follow-up period, the issued pressure could be increased by trans-scrotal injection of saline solution into the compensation pouch to improve continence status. The option to increase the issued pressure in situ was not considered as a revision.
RESULTS
Twenty-seven male patients with moderate- to-severe UI were treated by implantation of the ZSI 375 AUS between September 2013 and April 2016 and then followed up to April 2017. Patient age at the surgery ranged from 55–78 with a mean age of 67.70 years old. At presentation, 8 patients (29.63%) had moderate incontinence and 19 patients (70.37%) had severe incontinence: mean 5.15 pads per day (range: 3–10 pads per day).
The aetiologies of incontinence were radical prostatectomy in 26 patients (96.30%) and radical prostatectomy with adjuvant radiotherapy in one patient (3.70%). Two AMS 800 devices and one ZSI 375 device had been attempted previously in a total of three patients (11.11%). Three patients (11.11%) were also previously treated for urethral stricture. The ZSI 375 AUS was placed according to Staerman et al.’s9 surgical technique. The device was activated 8 weeks post-implantation. Twelve months after surgical procedure, 22 out of 27 patients (81.49%) presented a social continence and 2 out of 27 patients (7.41%) were improved. The success rate was 88.90%. Three out of twenty- seven patients (11.11%) were incontinent. Twenty-four months after surgical procedure, 14 out of 17 patients (82.35%) presented with social continence and 2 out of 17 patients (11.77%) were improved. The success rate was 94.12% and one patient (5.88%) was incontinent. Following 36-month follow-up five out of six patients (83.33%) presented social continence, no patient was improved so the success rate was 83.33%. One out of six patients presented incontinence (16.67%) (Table 1). Complications leading to permanent device removal arose in one patient (3.70%) with scrotal infection but without urethral erosion. This occurred within 1 month of AUS insertion and led to immediate removal.
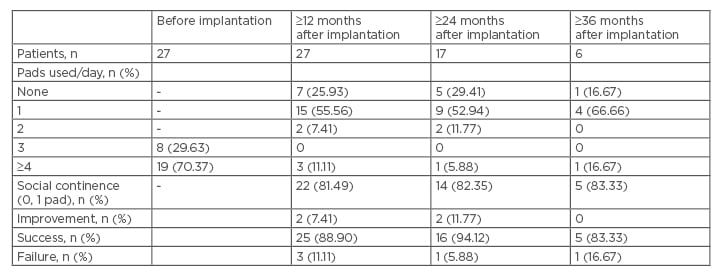
Table 1: Continence rates before and after device implantation.
n: number of patients.
Revision surgery was performed in a total of six patients (revision rate: 22.22%) and the patients enjoyed a social continence after revision. From these six patients, two (7.41%) presented with scrotal infection only, without any urethral erosion associated. The scrotal infections occurred more than 12 months after device insertion and led to an immediate removal of the device. One patient has undergone a new sphincter implantation 3 months after the removal and the second patient waited for 4 months after the removal of his first sphincter. Two patients (7.41%) presented with a urethral erosion. The last two patients (7.41%) from the ‘revision group’ presented with mechanical failure (Table 2). The patient who underwent pelvic irradiation and the patients with previous AUS did not present any adverse events.
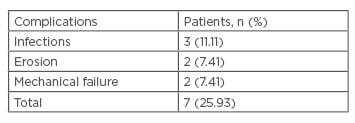
Table 2: ZSI 375 complications.
n: number of patients.
DISCUSSION
Many studies have assessed the outcomes of AMS 800 AUS safety and efficacy but direct comparison is not easy regarding the variability of definitions, perineal, and penoscrotal surgical techniques and selection of patients. The ZSI 375 was designed to simplify AUS preparation and surgical procedure because of the long learning curve needed to achieve mastery.
The capacity to increase or decrease the pressure was an advantage to reach a good rate of continence after device activation. After 12, 24, and 36-month follow-up, our social continence and success rates are in accordance with previous studies about ZSI 375 short-term results, which ranged from 87–94.2%9-11 and a continence rate for AMS 800 in a 24-month follow-up period of ≤90%.12,13
We are in line with the overall continence rate with AMS 800 AUS of 73% and the improved continence rate of 88%.14,15
In our study, the short-term complication rate was comparable to that achieved with AMS 800.12,14,16 ZSI 375 AUS infection occurred in three patients (11.11%). This high rate of infection is not in accordance with AMS 800 device series that range from 0.46–8.5% of cases.6,12,16,17 Although the implantation technic of the ZSI375 AUS is safe and simple, our infection rate, impacting the revision rate, should be improved with more experience. Urethral erosion was not the most frequently reported complication in the present study. Two patients (7.41%) presented with a cuff erosion, which was in accordance with the AMS cuff erosion rate of 4–12%.12,14,16,18 Drogo K. Montague19 proposed that urethral dissection and mobilisation during procedure could be responsible for early cuff erosion and urethral catheter insertion with poor deactivation of the AUS lead to late erosion. Our two erosions occurred 18 months after implantation of the AUS without any catheter insertion. Other factors such as blood perfusion in the urethra could explain our erosions.20-22 Mechanical failure in our series occurred in two patients (7.41%) 12 and 16 months after the surgical procedure. This was equivalent to the 6% of AMS 800 device mechanical failure with a mean follow-up of 36 months.6 However, in a report of 530 patients implanted with AMS 800 the 5 years Kaplan–Meier freedom from reoperation was 79.4% for primary cases and 88% for revision surgeries.7 The follow-up of our series is too short to be compared with the AUS of reference. It should be noted that the dissociation of total failure rate (Table 1) and the failure rate at 12, 24, and 36 months (Table 2) is due to revision. Clemens et al.17 reported a revision rate after AUS AMS 800 implantation during 5-year follow-up of 50%. Regarding this result, the revision rate of the ZSI 375 must be re-evaluated in the long-term with larger series. Our retrospective study has further limitations; it would have benefited from a prospective approach with patient satisfaction questionnaires and incontinence evaluation using pad weight as recommended by The International Continence Society (ICS) and not pad number.
CONCLUSION
We have described a preliminary clinical result of the AUS ZSI 375. The follow-up time was long enough to demonstrate that ZSI 375 offered acceptable and satisfactory urinary control with the usual AUS adverse events to patients with urinary stress incontinence. The option to adjust pressure in situ should reduce the need for surgical revision, but longer-term studies of safety and efficacy, as well as a comparison with other similar devices should be carried out to confirm these good preliminary results.








