Interview Summary
EMJ conducted an interview with Param Mariappan, who is a Consultant Urological Surgeon, Director of Edinburgh Bladder Cancer Surgery, at Western General Hospital Edinburgh, and an Honorary Professor at the University of Edinburgh, UK. Mariappan is a renowned clinician-researcher in the field of urological cancer, with a keen interest in improving the treatment experience and outcomes in patients receiving bladder cancer surgery. Mariappan sits on the European Association of Urology (EAU) Bladder Cancer Guideline panels, and the Core Committee of the International Bladder Cancer Group (IBCG), and is a medical advisor to the Fight Bladder Cancer charity.
In this interview, Mariappan discusses the importance of achieving appropriate benchmarks for the treatment of non-muscle-invasive bladder cancer (NMIBC), and evaluates the impact of transurethral resection of bladder tumour (TURBT) on recurrence and progression. He talks about his extensive research and recent publication findings, which aim to establish national quality performance indicator (QPI) programmes that include quality of detrusor muscle sampling and use of single instillation of chemotherapy (mitomycin C; SI-MMC) after TURBT for the improvement of outcomes in patients with bladder cancer.
INTRODUCTION
Bladder cancers are classified into muscle-invasive and non-muscle-invasive, the latter accounting for 75% of cases.1 Accurate and timely diagnosis and treatment are crucial, as bladder cancer can become life-threatening.1 However, there has been no significant improvement in survival rates in the last 30 years.2 The initial TURBT is vital for both diagnostic and therapeutic purposes, and facilitates the determination of prognosis. Mariappan, the lead author of a recent publication that assessed benchmarks on time to recurrence and progression in NMIBC, was interviewed.3
Could You Discuss the Current Guidelines for Non-muscle-Invasive Bladder Cancer Diagnosis and Treatment?
Around half of patients with NMIBC have low-grade non-invasive cancers (Stage Ta LG/G1).1 The other half have high-grade disease, which can either be non-invasive, or invasive (Stage T1 HG/G3).1 When you delve deeper into it, the distinction between the proportions of these types is largely based on historical data.1,4 The issue is that a proportion of what are deemed to be high-grade NMIBC are actually muscle-invasive, and these are potentially life-threatening. An element of my work aims to improve diagnostic accuracy through the initial TURBT so that these aggressive cancers are effectively and efficiently identified from the outset, and correct risk grouping can be ascribed.
Apart from the safety element, the initial TURBT is very important for two purposes. The first is to obtain information, identifying tumour characteristics: size, number, location, and presence of carcinoma in situ (CIS). The second is to clear the visible cancer and identify the disease extent, including CIS when possible. This can be augmented by optical enhancements; for example, photodynamic diagnosis or narrow-band imaging.5,6 So, when the case is presented to the multidisciplinary team (MDT), we have information for risk-adapted treatment selection.
How Important Is It to Stratify Patients Into Risk Groups?
The risk group determines what adjuvant treatment and surveillance regime they receive, including frequency of surveillance and upper tract imaging, which is recommended for high-risk and very high-risk groups.1
Patients are divided into low-, intermediate-, high-, and very high-risk (Table 1). The IBCG and EAU have different categorisation criteria (Table 1).1,7,8 Low-risk are solitary small tumours, pathologically low-grade and non-invasive, and require complete TURBT, followed by a single instillation of intravesical chemotherapy (SI-MMC in the UK).4 On the other hand, patients with high-risk, high-grade (Ta or T1) undergo TURBT; although guidelines may not recommend single instillation of chemotherapy, this may still be used because the grade or stage of the cancer would not be known at the time of the initial TURBT.1,7,8 Intermediate-risk are those who fall between these two groups. In the UK, patients get TURBT, single instillation, followed by a course of intravesical instillation, usually over 6 weeks, and are kept on close surveillance.4
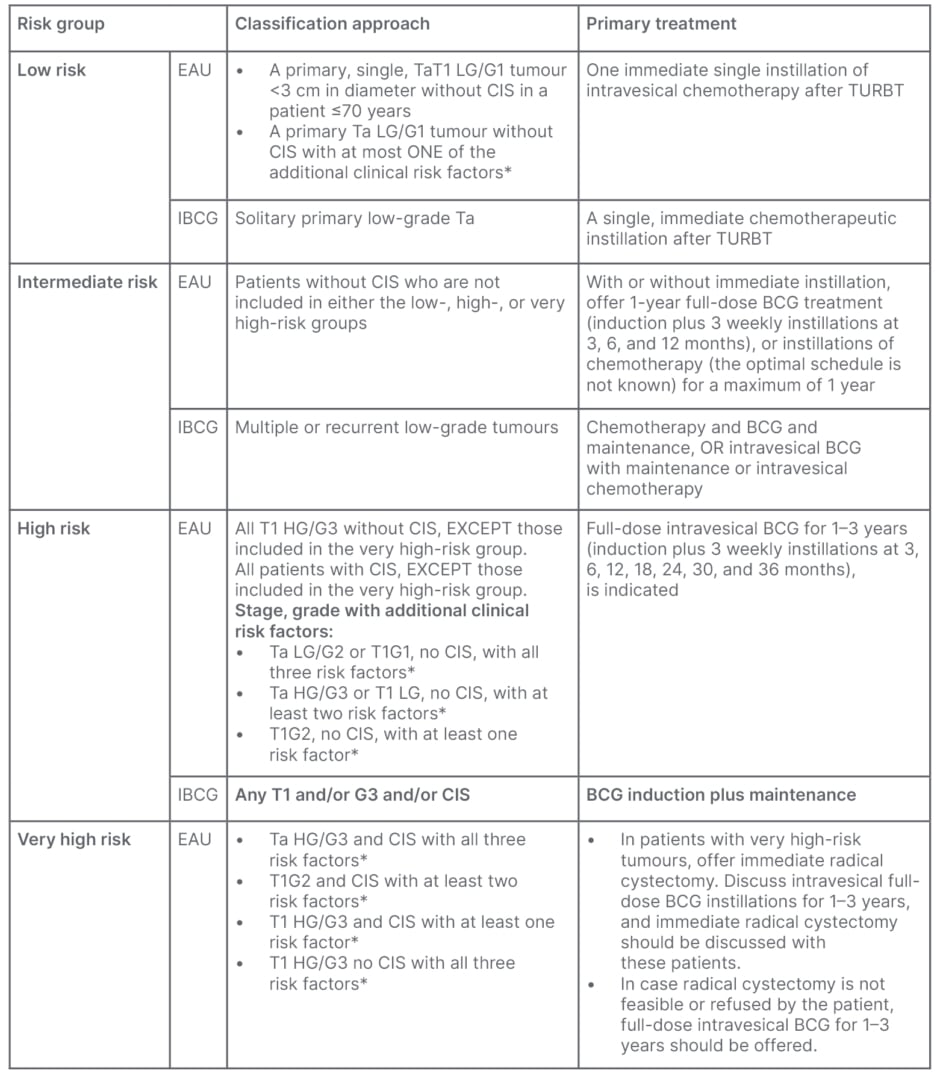
Table 1: Risk groups determined by classification approach and recommended primary treatment
per guidelines.1,7
*Additional clinical risk factors are: age >70; multiple papillary tumours; tumour diameter ≥3 cm. Carcinoma in situ cannot be managed by an endoscopic procedure alone, and should be offered either intravesical BCG instillations or radical cystectomy.
BCG: Bacillus Calmette-Guérin; CIS: carcinoma in situ; EAU: European Association of Urology; IBCG: International Bladder Cancer Group; N/A: not applicable; TURBT: transurethral resection of bladder tumour.
Are There Any Concerns with Current Practices, and What is the Adherence to Perioperative Care After Resection?
Prior to 2002, NMIBC management focused on single intravesical instillation, or adjuvant installation of chemotherapy to augment TURBT (Figure 1). A study published in 2002 demonstrated, for the first time, variance across centres of excellence, indicating that the quality of the initial resection played a significant role in recurrence rates.9 It became apparent that the surgeon had something to do with these outcomes being variable from centre to centre.9 As a result, I brought quality control and continuous quality improvement into the equation, with a search for surrogates and benchmarking.10 This research demonstrated that detrusor muscle sampling and surgeon experience were associated with lower recurrence rates;10 and we validated this work within two other cohorts, recommending a benchmark for good quality TURBT.11
Guidelines now highlight the importance of the quality of TURBT.12 The EAU emphasises the importance of detrusor muscle sampling in the appropriate patient, single instillation, and the use of the bladder diagram to document tumour characteristics (size, number, location, and appearance) and completeness of resection (including a TURBT checklist), which will be expanded in the 2024 Guideline Update.1
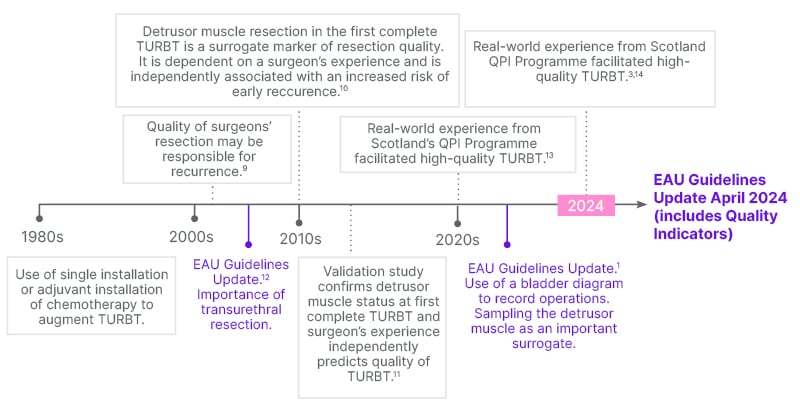
Figure 1: Timeline for evolution of the management of non-muscle invasive bladder cancer
in Edinburgh, UK.
EAU: European Association of Urology; TURBT: transurethral resection of bladder tumour; QPI: Quality
Performance Indicators.
There are barriers to the instillation of chemotherapy following TURBT. These challenges range from surgeons’ prejudices or lack of belief in the evidence, to lack of drug access or obtaining it from the pharmacy, delays in prescribing, fear of bladder perforation, and a lack of standard pro forma that describes the need for instillation.15
RECENTLY YOU PUBLISHED AN ARTICLE ON ACHIEVING BENCHMARKS FOR NATIONAL QUALITY INDICATORS
What Was Your Primary Motivation Behind This Research?
When I began as a consultant, I noticed that patients with bladder cancer were not necessarily managed in a way that provided comprehensive information for their diagnosis. The information available to the MDT tended to be patchy, and it was observed that higher recurrence rates and progression were not only associated with muscle-invasive cancer.
In 2005, I conducted a prospective performance audit based on a simple, reproducible, objective surrogate measure: the sampling of the detrusor muscle. This was done to evaluate the surgeon’s performance, and determine if there was any association between detrusor muscle sampling, experience, and recurrence rate.10 This work led us to consider introducing benchmarks to assess the quality of TURBT.11
Performing the initial operation properly and thoroughly; documenting everything, including a standard description of the tumour; use of a standardised pathology checklist, describing grade, stage, depth of invasion, variant histology, and the presence or absence of CIS, among others;16 and an MDT overview, are quality elements that improve standards of care. Additionally, administering a single instillation of chemotherapy afterwards, and selectively performing re-resection, are very important factors.
We introduced these standards into Scotland’s Quality Performance Indicators (QPI) programme in 2014; and in 2020, we published evidence behind the recurrence rate at first check cystoscopy, consequent to the QPI programme.13 In 2024, we described 5-year recurrence and progression outcomes from this cohort.3
How Were Targets (Benchmarks) Derived or Defined for the Quality Performance Indicators for Non-muscle-Invasive Bladder Cancer?
To improve treatment outcomes, a benchmark was established using our five-factor quality measure,11 the ‘pentafecta’ (Table 2).11,14,17

Table 2: In-house ‘pentafecta’ quality performance indicator benchmarking.11,14,17
TURBT: transurethral resection of bladder tumour.
I planned to introduce these benchmarks as standards to the regional South East Scotland Cancer Network (SCAN);10 however, this fortuitously coincided with the Scottish Government’s Better Cancer Care policy,18 through which we had the QPI programme. I was fortunate to lead the QPI programme development,14 to improve cancer outcomes,10,17 unify standards, and reduce variability across the country.3,14,18
What Key Outcomes Were Identified, and What Significance Do These Have to Clinical Practice?
These benchmarks were developed to improve outcomes, and to allow more precise risk stratification by the MDT, which enables us to offer adjuvant treatment accordingly.10,11
Two key quality indicators emerged that could influence recurrence and progression: meeting the hospital target for sampling detrusor muscle, and performing a single instillation of chemotherapy.14
We also found, for the first time to my knowledge, a significant reduction in cancer progression associated with the SI-MMC, based on the newest classification of progression (from Ta to T1, G1 to G2, low-grade to high-grade, or G2 to G3).3
We introduced ‘tolerance’ into our targets for situations where certain procedures may not be appropriate. Centres meeting the 80% target of detrusor muscle sampling showed significantly better recurrence and progression risk.3 Also, SI-MMC was associated with a 20.4% reduction in recurrence rate and progression.3 The audit and feedback mechanism within the QPI programme improved performance over time.3,10,11
Can You Elaborate on the Approaches Adopted During Your Research, and How They Have Evolved?
Data collection, ensuring safety, dedicated clinicians, and experience are as important as meeting benchmarks. We review and modify the QPIs every 3 years based on performance data, and emerging evidence.14 In patients with low-grade, small, non-invasive tumours; thin-looking bladders; or small tumours in the elderly, detrusor muscle sampling is not always necessary, and could be dangerous. The denominator for measuring the target for detrusor muscle sampling has shifted from ‘all NMIBC’ to only patients with high-grade NMIBC.19 Similarly, SI-MMC after initial resection is not recommended for patients with a thinned-out or perforated bladder, or if the patient has had bleeding or muscle-invasive cancer. Our new target is 80% use of a SI-MMC following initial TURBT for low-grade non-invasive cancer, up from 60%.19
How Do Quality Indicators Impact Tumour Progression and Recurrence?
The European Organisation for Research and Treatment of Cancer (EORTC) risk calculators20 have shown that if someone has prior recurrence, their prognostic risk of progression is higher.1 I think that, by reducing recurrence with SI-MMC and identifying risk groups, we implement measures to reduce progression. Currently, we have a large database of patient information, which allows for detailed analysis of factors contributing to the reduction in progression.
Secondly, the definition of cancer progression in previous studies was limited to muscle-invasive cancer. We have expanded to include lesser levels of cancer progression (low-grade to high-grade, Ta to T1, or G1 to G2 or G3) proposed by the IBCG.7
WHAT ARE THE IMPLICATIONS FOR ACHIEVING BENCHMARKS IN FUTURE PRACTICE?
From a Scottish context, centres that do not adhere to the evidence-based recommendations will hopefully take note of our audit from Scotland, and strive not to be the outlier.3 This approach also empowers patients and the public by providing them with information.
Future clinical trials should utilise centres of excellence that meet specific benchmarks based on their performance in NMIBC. We are introducing a new dimension: considering the audit process, and examining our own practice.10,11 We hope this will reduce the variability between centres that was previously described.9 These are simple interventions that can influence outcomes.
By using a QPI programme, we are constantly assessing performance and providing feedback. This encourages continuous improvement. It’s about constantly trying to improve, and using surrogates as our benchmarks.11,13 Only then will we continue to improve patient outcomes. We are providing the evidence to support the use of these benchmarks. We have now shown that these benchmarks translate to real-world clinical outcomes.
WHAT ARE YOUR NEXT STEPS AND FUTURE VISION FOR BLADDER CANCER CARE?
Implementing QPI programmes can aid treatment outcomes, peer comparisons, and, potentially, clinical trials involved in future drug development. Future steps include using benchmarks in NMIBC within a novel risk calculator.3 In addition, we are evaluating the necessity of a re-resection, in whom to use SI-MMC, and exploring biomarkers for the management and surveillance of bladder cancer.
The use of benchmarks, quality control, and continuous quality improvement are crucial for improving patient outcomes. Everyone in the bladder cancer care community has a part to play, making it a way of life. The initial TURBT is the most important part of the patient’s journey for bladder cancer. When done properly, it can avoid unnecessary repeat surgery, save time, and improve survival. A risk stratification system can help determine the most appropriate treatment pathway. In my opinion, one of the best ways to benchmark a urology service is by how well they manage patients with NMIBC. The quality of a urology centre could be judged by the quality of its TURBT, such as lower (<10%) early recurrence rate at the first follow-up cystoscopy at 3 months.13







