In real-world clinical practice, the decision to permanently implant a patient with a sacral neuromodulation system is often dependent on a mix of diary-based symptom improvement, patient satisfaction, and quality of life benefits, whereas therapy response is usually defined based on diary improvements alone. In this abstract, the authors reported on therapy responder rates within 1 year after implantation in overactive bladder (OAB) patients, as well as in the urinary urge incontinence (UI) and urinary frequency (UF) subgroups, and evaluated how the criteria for permanent implantation affect response measured during follow-up. This analysis was performed post hoc using data from SOUNDS: a prospective, multicentre, observational study to evaluate clinical effectiveness, quality of life, and safety of sacral neuromodulation with the InterStim™ system for urinary voiding dysfunctions during a 5-year follow-up in a real-life setting.
Patients suffering from OAB and non-obstructive urinary retention were enrolled. Site selection aimed to represent the French market regarding volume and type of institution. Therapeutic success in OAB patients with UI or UF was defined similar to definitions used by Siegel et al.:1 ≥50% reduction in average leaks/day for UI, and ≥50% reduction in voids/day or a return to normal voiding frequency (<8 voids/day) for UF. Data were reported following intention-to-treat principles without missing data imputation at two follow-up visits (at an average of 3.1 and 10.0 months after implantation).
Overall, 320 patients were enrolled from 25 sites, and 247 were permanently implanted. Patients were predominantly female (84%) with a mean age of 60.5 years (standard deviation 15.1 years). Enrolled patients were affected by OAB (91%) or non-obstructive urinary retention (9%), and tested/implanted patients were scheduled for a de novo (78%) or a replacement of an existing InterStim™ system (22%) procedure.
Figure 1 shows responder rates in follow-up visits for two groups of de novo patients: patients that were implanted without any specific test response criteria defined, at the discretion of the sites; and a subset of patients that would have received the permanent implant, based on their voiding diaries at the end of the test phase meeting responder criteria defined above.
Responder rates in SOUNDS are similar to what has been previously published.1-3 These data suggest that patients responding at the end of test stimulation, based on voiding diaries, tend to have greater therapeutic success in follow-up visits. A further predictor analysis to evaluate the effect of response during test stimulation on therapeutic success at long-term follow-up is needed to confirm these results.
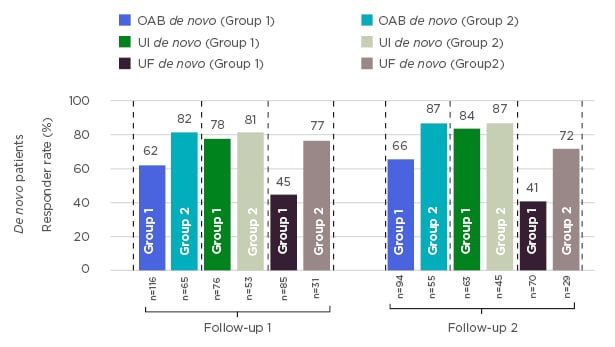
Figure 1: Response rates in patients permanently implanted with an InterStim™ sacral neuromodulation system.
OAB: overactive bladder; UF: urinary frequency; UI: urinary incontinence.








