According to the European Association of Urology (EAU), 20≥30% of adults experience urinary incontinence, while up to an estimated 30% of females aged 30–60 years experience urinary incontinence.1 Those patients who are identified as having an overactive bladder (OAB) suffer urgency or even urinary urgency incontinence, which are initially treated with medication. In spite of improved drug delivery systems and pharmacological advancements, this treatment remains refractory to medical treatments.
At the next level the EAU guidelines suggest onabotulinumtoxin A, sacral nerve modulation, or percutaneous tibial nerve stimulation;1 however, the limited lasting effect of onabotulinumtoxin A2 requires that patients need repeated injections into the detrusor muscle. Similarly, patients see disadvantages with sacral nerve modulation because of the surgery required to implant the electrodes into the gluteal and battery maintenance issues. Furthermore, implants limit the ability to do diagnostics, such as magnetic resonance imaging (MRI).3 In recent years, percutaneous tibial nerve stimulation has become available, but the stimulation had to be performed in the office once a week for 20 minutes.4,5
With a chronic implant (Figure 1) and an external device (Figure 2), the patient can become more independent, without almost any diagnostic restrictions. The tibial nerve stimulation via the sacral plexus is known to control bladder function, but the actual mechanism of action has still not yet been identified.6 We report our preliminary experience with a minimally invasive, implantable wireless neuromodulation system to perform chronic tibial nerve modulation.7
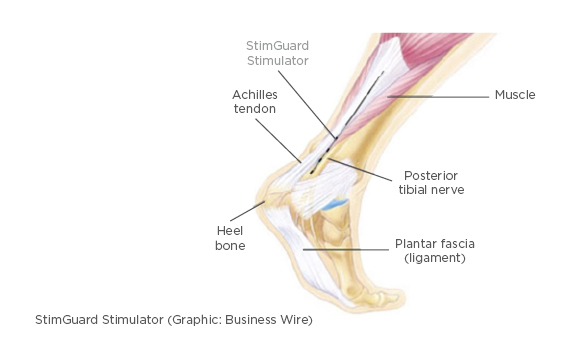
Figure 1: Drawing of the location of the chronic implant located close the post-tibial nerve.
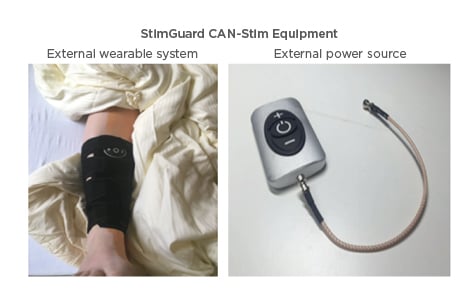
Figure 2: Positioning of the external device (picture on the right) in a sleeve at the ankle, where the implant is positioned during stimulation (Figure 1).
The external device is connected to an external antennae, which is positioned above the internal antenna of the implant. Through the antennas energy and the programming (like frequency, amplitude, pulse width) is transported. The patient is able to switch the device on/off and increase or decrease the amplitude.
The primary inclusion criteria was adult patients with refractory OAB without obstructive/ infective lower urinary tract and not taking any anti-muscarinic and beta-3 adrenergic agonists. From two centres, 21 patients were enrolled and 4 were excluded. After informed consent, the electrode was implanted in the leg along the tibial nerve 4–7 cm above the medial malleolus through a 5 mm skin incision in local anesthesia. The wireless Nautilus antenna assembly was the program used to control the stimulation parameters. Patients were asked to activate the implant on a subsensory threshold during the evening or while sleeping (8 hours).
All enrolled patients tolerated the procedure well. At the end of the 60–90-day follow-up, 5 patients had lead migration, 2 required revision, and other implants were removed. In 1 patient, the implant seemed not to cause any effect, independent of the amplitude.
During the early follow-up period, significant improvement in Incontinence Quality of Life (I-QOL) and Overactive Bladder Questionnaire (OABq) scores were observed (p<0.05). In addition, urgency incontinence episodes, Global Response Self-Assessment (GRS), and Patient Perception of Intensity of Urgency Scale (PPIUS) demonstrated a significant improvement. Although the mean number of voids remained unchanged, the mean voided volume increased by 70 mL. Those who suffered from nocturia normalisation reduced to an average of <1 incident per night. By the end of the 90-day evaluation, I-QoL and OABq scores reflected a delayed but significant improvement.
This initial pilot study demonstrates that chronic tibial nerve modulation is safe and effective with encouraging results. Surgical and implant modifications have been made to ensure that the chronic implant remains in its optimal position. US Food and Drug Administration (FDA) studies have been initiated due to these encouraging results and plans are underway to verify the findings on a larger scale.








