BACKGROUND AND AIMS
The Acute Cystitis Symptom Score (ACSS) is a patient self-reporting questionnaire for the clinical diagnostics and patient-reported outcome that may assess the symptoms and the effect on the quality of life in women with acute uncomplicated cystitis.1,2 The current study aimed to create a validated Spanish version of the ACSS questionnaire. The questionnaire was developed in Russian and English by the European Association of Urology (EAU) Section of Infections in Urology (ESIU).1
MATERIALS AND METHODS
The ACCS questionnaire was translated from its original Russian and American English into Spanish by translators with Spanish as their mother language. Then, four urologists from Spain and Latin America reviewed the two translations and established a consensus form. This consensus form was then translated back into Russian by a translator with Russian as their mother language and American English by a translator with American English as their mother language, in order to check and exclude any relevant change of the meaning. This consensus version was then used for all countries with Spanish as their primary language for cognitive assessment processes. All comments were considered, and the study version was established.3–7 The study version was used for clinical validation in female patients from Spain, Colombia, and Peru.
Clinical evaluation was carried out by 198 female participants (151 with symptoms suspicious of uncomplicated cystitis and 47 controls). Psychometric characteristics of the ACSS were tested using coefficients of Cronbach’s α and split-half reliability. The diagnostic characteristics were tested using specificity, sensitivity, diagnostic odds ratio, positive and negative likelihood ratios, area under the receiver operating characteristic diagnostic accuracy, and risk ratio. Average point estimates and 95% confidence intervals were used to present the results of the tests.
RESULTS
The age of the 151 patients (mean: 49.9 years; standard deviation [SD]: 18.6) and 47 control (mean: 53.2 years; SD: 19.0) and their additional conditions at baseline visits such as menstruation, premenstrual syndrome, pregnancy, menopause, and diabetes were compared. Among the isolated pathogens, the most frequent was Escherichia coli, present in 94 urine cultures (74.6%). Other micro-organisms isolated were Klebsiella pneumoniae in 7 cases (5.5%), Proteus mirabilis in 7 (5.5%), Group B Streptococcus in 4 (3.1%), Enterococcus faecalis in 3 (2.3%), Staphylococcus saprophyticus in 3 (2.3%), and others.
ACSS comparative analysis reported a significant difference (p<0.001) between patients and controls at the baseline visit regarding sum score of the typical symptoms (mean: 8.11; SD: 4.31; and mean: 0.70; SD: 1.04), differential symptoms (mean: 1.46; SD: 2.1; and mean: 0.28; SD: 0.58), and quality of life (mean: 4.9; SD: 2.1; and mean: 1.32; SD: 1.9) (Table 1). At the follow-up visit, in the ‘Evolution’ domain of Part B of the questionnaire, 74.2% of patients were asymptomatic (back to normal), 15.7% much better, and 10.1% somewhat better. None of the patients persisted without clinical changes or had worsened in their symptoms
Cronbach’s α of the ACSS was 0.88 (mean: 0.85; SD: 0.90), and the split-half reliability was 0.89 (mean: 0.78; SD: 0.93). Using a sum score of >6 for typical symptoms, a specificity of 0.98 (mean :0.89; SD: 1.00) and sensitivity of 0.53 (mean: 0.54; SD 0.79) were found. The diagnostic odds ratio was 16.33 (mean: 2.08; SD: 128.06); diagnostic accuracy 0.56 (mean: 0.44; SD: 0.62); positive likelihood ratio and negative likelihood ratio were 25.57 (mean: 3.65; SD: 179.13) and 0.48 (mean: 0.39; SD: 0.58), respectively; risk ratio was 1.80 (mean: 1.51; SD: 2.14); and area under the receiver operating characteristic was 0.73 (mean: 0.68; SD: 0.77).
CONCLUSIONS
The validated Spanish ACSS questionnaire is a reliable, valid, and easy-to-use questionnaire that can evaluate the symptoms and clinical outcomes of patients with acute cystitis. It can be used as a patient’s self-diagnosis of acute cystitis, as a patient-reported outcome measure tool and help to rule out other pathologies in patients with voiding syndrome.2-7
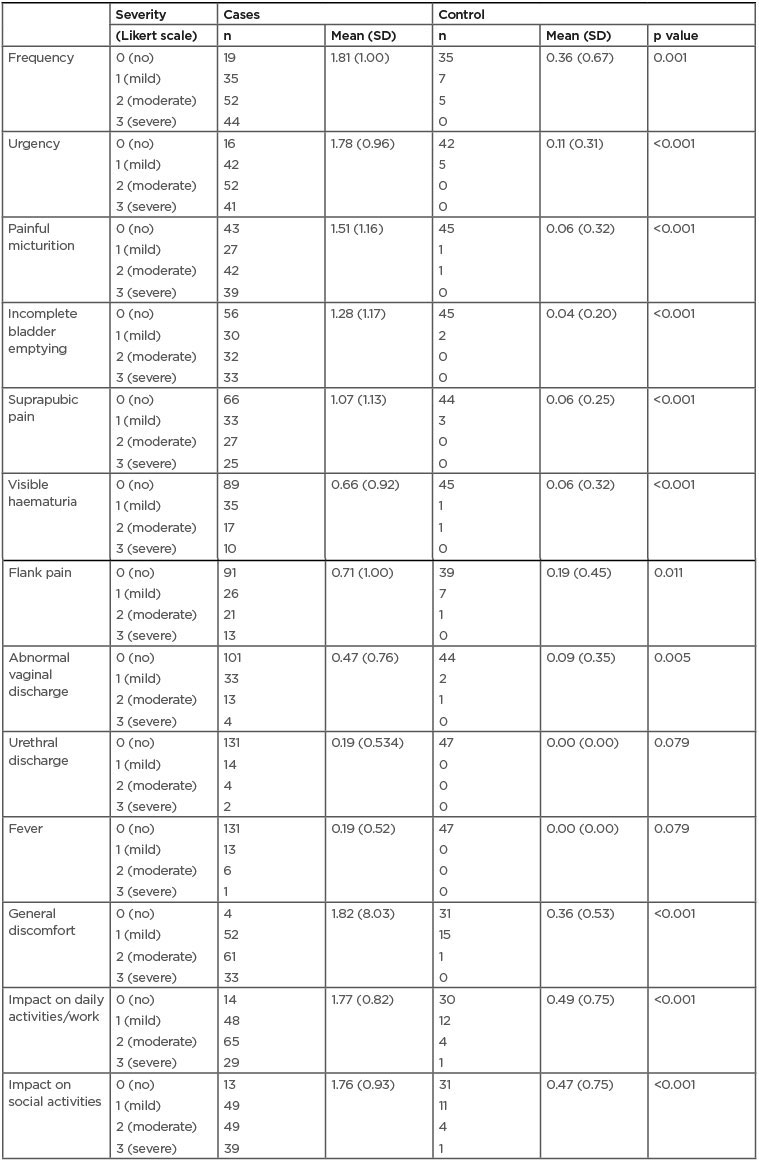
Table 1: Acute Cystitis Symptom Score questionnaire scores for each item of the domains: typical symptoms, differential, and quality of life for cases and controls.
SD: standard deviation.








