Lower urinary tract symptoms (LUTS) affect ˜44% of males worldwide1 and can negatively impact quality of life (QoL).2 Inadequate response with monotherapy can occur in up to two-thirds of men with LUTS and often requires add-on therapy (e.g. antimuscarinic drug);3 however, treatment with add-on therapy has not been established in routine clinical practice. Vesomni™ is a fixed-dose combination tablet containing 6.0 mg solifenacin (antimuscarinic) and 0.4 mg tamsulosin (alpha-blocker) used to treat LUTS associated with benign prostatic hyperplasia (LUTS/BPH). EUROPA is a 1-year observational study evaluating the impact of Vesomni on QoL and treatment satisfaction in routine clinical practice.
Men with LUTS/BPH who were not adequately responding to monotherapy and who were prescribed Vesomni in routine clinical practice were enrolled in ˜59 sites throughout Belgium, Czech Republic, Portugal, Slovenia, Spain, and the UK. Clinical findings, medications for LUTS/BPH, and treatment-emergent adverse events (TEAEs) were collected for 1 year before and after Vesomni was prescribed. Patient-reported outcomes included the Overactive Bladder Questionnaire (OAB-q), International Prostate Symptom Score (IPSS), treatment satisfaction visual analog scale (TS-VAS), and European QoL Five Dimensions Questionnaire (EQ-5D-5L). These were recorded at baseline (Visit 1), Weeks 4–8 (Visit 2), and Weeks 9–18 (Visit 3). The primary endpoint was the change from baseline in symptom bother, measured by OAB-q (0=no bother; 100=very great deal of bother). Key secondary endpoints included change from baseline in total and coping, concern, sleep, and social interaction subscale scores of the OAB-q (0=very great deal of bother; 100=no bother), treatment satisfaction via the TS-VAS (0=not at all satisfied, 100=completely satisfied), total IPSS (0=no symptoms; 35=most severe symptoms), IPSS QoL (0=delighted; 6=terrible), and health status via EQ-5D-5L (0=worst health, 100=best health). A 10-point improvement in any OAB-q subscale score was considered clinically meaningful;4 a 3-point improvement in total IPSS was considered clinically meaningful; a 0.5-point improvement was considered the minimal clinically important difference for IPSS-QoL.5,6 This interim analysis included patients who completed ≥3 months of observation while receiving Vesomni (through Visit 3); 298 were evaluated for safety/tolerability; 251 were evaluated for QoL and treatment satisfaction.
Treatment with Vesomni yielded clinically meaningful improvements in OAB-q symptom bother score at Weeks 4–8 and 9–18, total and coping, concern, and sleep subscale scores at Weeks 4–8, and total and coping and sleep subscale scores at Weeks 9–18 (Figure 1). A clinically meaningful improvement in TS-VAS was also observed by Weeks 4–8 and was maintained through Weeks 9–18 (Figure 2). Health status via the EQ-5D-5L improved modestly from a mean of 65.2 at baseline to 72.1 at Weeks 4–8 and 69.8 at Weeks 9–18. Total IPSS score improved from 15.5 at baseline to 10.6 at Weeks 4–8 (mean of 4.7 points improvement) and to 10.9 at Weeks 9–18 (mean of 3.5 points improvement). IPSS-QoL scores improved by 1.2 points at Weeks 4–8 and by 0.9 points at Weeks 9–18; all improvements in IPSS scores were clinically meaningful. One hundred and fifty-one TEAEs were reported in 68 patients (22.8%); 131 (87.0%) were mild-to-moderate in severity. The most common TEAEs were constipation (6.0%), dry mouth (5.4%), and dyspepsia (3.0%); no deaths, serious adverse events, or cases of urinary retention requiring catheterisation were reported.
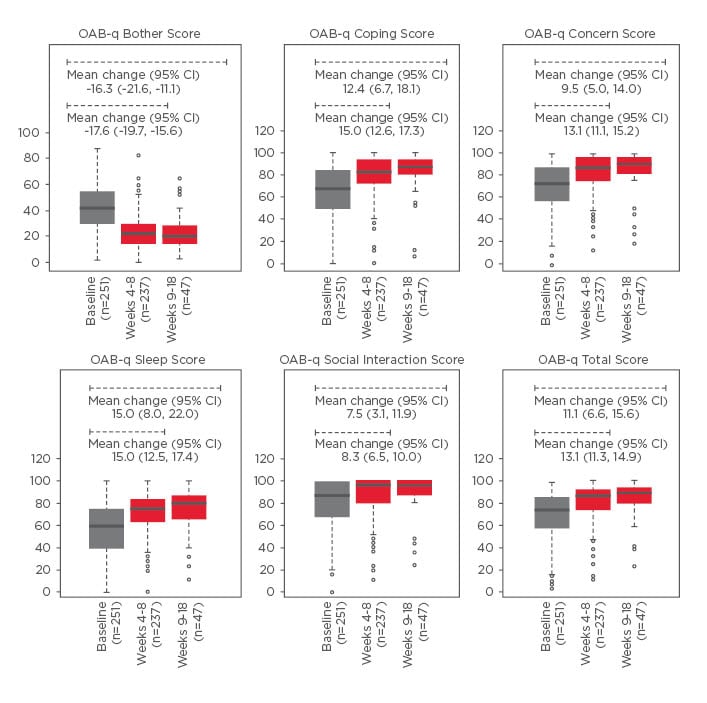
Figure 1: Primary endpoint: Mean change in OAB-q scores.
Boxplots depict the median (solid lines), interquartile range (box), minimum and maximum (dashed lines), and outliers (circles).
A decrease in OAB-q bother score is defined as an improvement. An increase in all OAB-q subscales and total score is an improvement.
CI: confidence interval; OAB-q: Overactive Bladder Questionnaire.
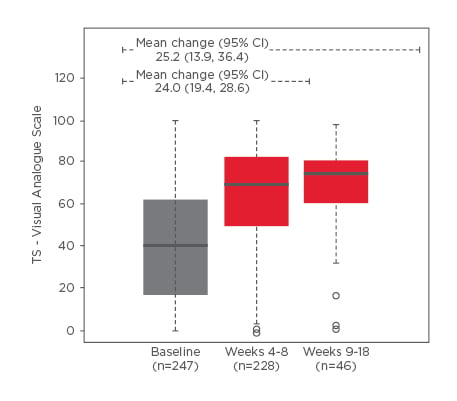
Figure 2: Treatment satisfaction scores on the visual analog scale.
Boxplots depict the median (solid lines), interquartile range (box), minimum and maximum (dashed lines), and outliers (circles). A higher TS-VAS score indicates greater treatment satisfaction.
CI: confidence interval; TS-VAS: treatment satisfaction visual analog scale.
EUROPA is the first report of the treatment benefit and safety/tolerability of Vesomni in routine clinical practice; it demonstrated clinically meaningful improvements in health-related QoL and treatment satisfaction. These results support Vesomni as a treatment option for men with LUTS/BPH not adequately responding to monotherapy.








