INTRODUCTION
Healthcare-associated infections (HAIs) in urological patients have special characteristics due to specific risk factors such as a high prevalence of urinary catheterisation and as surgery is commonly performed during hospitalisation.1-3 Furthermore, an adequate knowledge of the microbiological patterns is of paramount importance to optimise the outcomes.4,5 Our purpose was to review the incidence and characteristics of HAIs in patients admitted in a urology department.
MATERIAL AND METHODS
We carried out a prospective observational study over 5 years (November 2011–September 2016) evaluating the incidence, types of HAIs, risk factors, microbiological characteristics, and patterns of susceptibility to antibiotics among patients admitted to our urology ward.
RESULTS
Out of 8,138 patients, 518 (6.4%) experienced some type of HAI. The evolution in the incidence of HAIs over the time is shown in Figure 1. The most common types were urinary infections (70.4%) followed by surgical site infections (21.7%). Factors associated with a higher risk of HAIs were older age (odds ratio [OR]: 1.009; p=0.002), male sex (OR: 1.225; p=0.017), arterial hypertension (OR: 1.213; p=0.033), heart disease (OR: 1.244; p=0.043), liver disease (OR: 1.492; p=0.035), prior urinary infection (OR: 3.402; p<0.001), immunosuppression (OR: 2.058; p<0.001), American Society of Anaesthesiologists (ASA) Score III-IV (OR: 1.298; p<0.001), and an indwelling urinary catheter prior to admission [OR: 1.987; p<0.001] or during hospitalisation (OR: 1.379; p<0.001). Multivariate analysis confirmed several risk factors: immunosuppression (OR: 1.735; 95% confidence interval [CI]: 1.138–2.646; p=0.010), ASA Score III-IV (OR: 1.217; 95% CI: 1.063-1.392; p=0.004), prior urinary infection (OR: 2.648; 95% CI: 1.161–6.041; p=0.021), and an indwelling urinary catheter prior to admission (OR: 1.838; 95% CI: 1.427–2.366; p<0.001). Surgical procedures associated with the highest incidence of HAIs were open prostatic surgery (7.2%), renal surgery (7.2%), and radical cystectomy (51.6%).
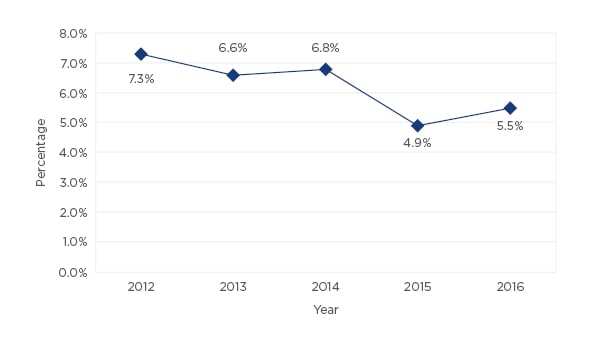
Figure 1: Evolution in the incidence of healthcare-associated infections in our urology department from 2012–2016.
The most commonly isolated pathogens were Escherichia coli (25.6%), followed by species of Enterococcus (18.2%), Klebsiella (13.8%), and Pseudomonas (11.7%). Staphylococcus aureus and Candida were isolated in 6.1% and 5.1% of positive cultures, respectively (Figure 2). No microorganisms were isolated in 28.4% of patients. E. coli showed resistance rates of 50% for fluoroquinolones, 10.9% for amikacin, and 1.9% for carbapenems. Klebsiella spp. showed resistance rates of 44.8% for third-generation cephalosporins, 39.7% for fluoroquinolones, and 6.8% for carbapenems. Among cultures where E. coli was isolated, 26.9% were extended-spectrum betalactamase (ESBL)-producing bacteria; this figure was 42.4% when Klebsiella was isolated. Enterococcus was the most commonly isolated micro-organism after cystectomy and showed resistance rates of 50.7% for fluoroquinolones and 1.3% for vancomycin. Pseudomonas aeruginosa showed resistance rates of 52% for quinolones, 34% for carbapenems, and 22% for amikacin.
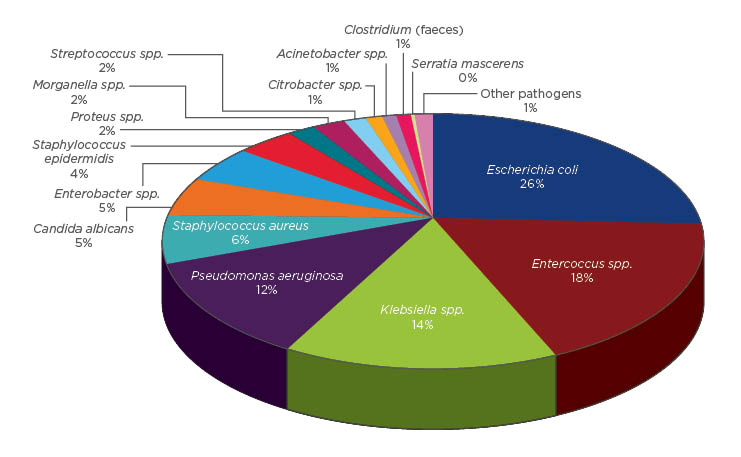
Figure 2: Micro-organisms isolated in patients with healthcare-associated infections by percentage.
Among patients with prior urinary infections, the incidence of HAIs was 18.5% and the most commonly isolated micro-organism was Klebsiella, which represented 28.2% of positive cultures and 72.7% were ESBL-producing Klebsiella. Among those with immunosuppression, the incidence of HAIs was 11.8%, and the most commonly isolated micro-organisms were Klebsiella (31.6%), E. coli (21.1%), and Enterococcus (18.4%).
DISCUSSION
Observational studies reviewing HAIs are required in order to decrease the incidence and to optimise the management of infections.4,6,7 Our series showed that being aware of HAIs may reduce HAIs from 7.0% to 5.5%. Furthermore, observational studies permit the determination of risk factors for infections and microbiological patterns in each patient and therefore enable the empirical antibiotic treatment to be tailored according to the characteristics of the patient.8-10
In conclusion, the risk factors related to HAIs were comorbidities, prior urinary infection, and a urinary catheter before admission. Although E. coli was the most frequently isolated micro-organism, Enterococcus, Klebsiella, and P. aeruginosa were commonly found. ESBL-producing Enterobacteriaceae occurred in 26.9% of cultures with E. coli and 42.4% of cultures with Klebsiella.








