Alpha-1 blockers have been used for the first-line treatment of benign prostatic hyperplasia (BPH).1 The phosphodiesterase 5 inhibitor tadalafil has recently been used for the treatment of BPH.2 The combination therapy or add-on of an anticholinergic or β3-adrenoceptor agonist (mirabegron) with α1-blocker is recommended for the treatment of BPH with overactive bladder (OAB).3 However, the efficacy of add-on treatment of mirabegron with tadalafil has not been reported.
In this study, the authors evaluated the efficacy and safety of the add-on treatment of mirabegron (50 mg/day) for OAB and BPH patients who were not satisfied with tadalafil (5 mg/day) monotherapy for ≥8 weeks. Male BPH patients with remaining OAB symptoms following tadalafil administration for >8 weeks were randomly assigned to either a tadalafil monotherapy (5 mg/day) group (TG) or a tadalafil and mirabegron combination therapy group (TMG), and were followed for 12 weeks. The primary endpoint was a change from baseline in total overactive bladder symptom score (OABSS). The secondary endpoints were changes in each question of OABSS, international prostate symptom score (IPSS), quality of life index, and micturition chart variables (number of voids per 24 hours, number of urinary urgency episodes per 24 hours, and number of urgency incontinence episodes per 24 hours).
A total of 176 patients were randomised to either TG (87 patients) or TMG (89 patients). The total OABSS (95% confidence interval) of the TMG at 12 weeks was significantly decreased by 1.78 (1.05–2.50) more than that of the TG (p<0.00001). Changes in night-time voiding score, urgency score, urgency incontinence score, IPSS total score, IPSS storage subscores, and National Institutes of Health chronic prostatitis symptom index voiding subscores were significantly greater in TMG compared to TG at 4 and 12 weeks after the treatments (p<0.05). The change in the number of voids per night, the number of voids per 24 hours, and the number of urgency episodes per 24 hours was significantly reduced in the TMG compared to the TG (p<0.05). One severe adverse event (1.2%), pain in the hip joint, was noted in the TG, and seven mild adverse events (8.1%) in the TMG.
In conclusion, the effect of tadalafil and mirabegron combination therapy on relieving OAB symptoms appeared to be greater than that of tadalafil. The present study is the first report on treatment with phosphodiesterase 5 inhibitor and β3 agonist combination regimen.
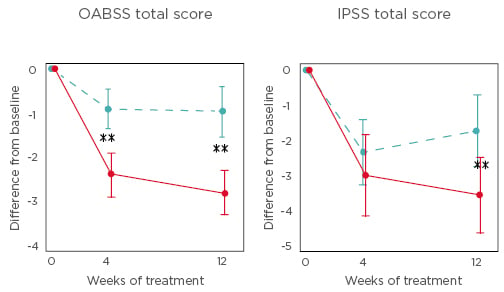
Figure 1: Changes in total overactive bladder symptom score and total international prostate symptom score from baseline to Week 4 and Week 12 of treatment.
Dotted blue line represents the tadalafil monotherapy group and the red line represents the tadalafil and mirabegron combination therapy group.
**p<0.01.
IPPS: international prostate symptom score; OABSS: overactive bladder symptom score.








