Meeting Summary
Prof Taylor opened the meeting and briefly highlighted the epidemiology of pregnancy in women with chronic rheumatic diseases (CRD). Dr Moltó presented the importance of disease control in women of childbearing age and various factors influencing fertility. Prof Nelson-Piercy addressed the need for a patient-centred multidisciplinary approach at each stage of pregnancy and how new clinical data may further inform current recommendations on the treatment of women with CRD. Dr Clowse concluded the meeting by reviewing the postpartum treatment recommendations for managing disease flares in this patient population.
Welcome and Introduction
Professor Peter Taylor
Rheumatic diseases are more common in women than in men,1 and disease onset tends to overlap with peak reproductive life (18–45 years).2 Therefore, family planning is an important clinical issue in CRD. For example, axial spondyloarthritis (axSpA) usually occurs between 15–30 years of age, is known to have delayed diagnosis, and of the total axSpA population, approximately 15–20% are women of childbearing age.3 These women were found to have a higher prevalence of emergency and elective caesarean section, pre-term delivery, and small-for-gestation-age pregnancy relative to healthy women.4 Patients with axSpA are less likely to experience symptom improvement during pregnancy and are more likely to relapse postpartum compared with patients with rheumatoid arthritis (RA).5
In 2016, a European League Against Rheumatism (EULAR) taskforce developed four overarching principles on the use of anti-rheumatic drugs before pregnancy and during pregnancy and lactation:
- Addressing family planning in young women as part of a treatment plan.
- Aiming towards control of disease activity whilst ensuring no harm to the fetus.
- Balancing the risk of treatment for the fetus versus the risk of untreated disease for the mother and fetus.
- Making shared, multidisciplinary treatment decisions that include the patient.6
Disease Control and the First Steps on the Road to Pregnancy
Doctor Anna Moltó
Women with CRD require a pre-pregnancy treatment plan, the aim of which is to achieve disease control and reassess treatment at the time of conception.6 Various factors relative to CRD and treatment influence the fertility of young female patients.
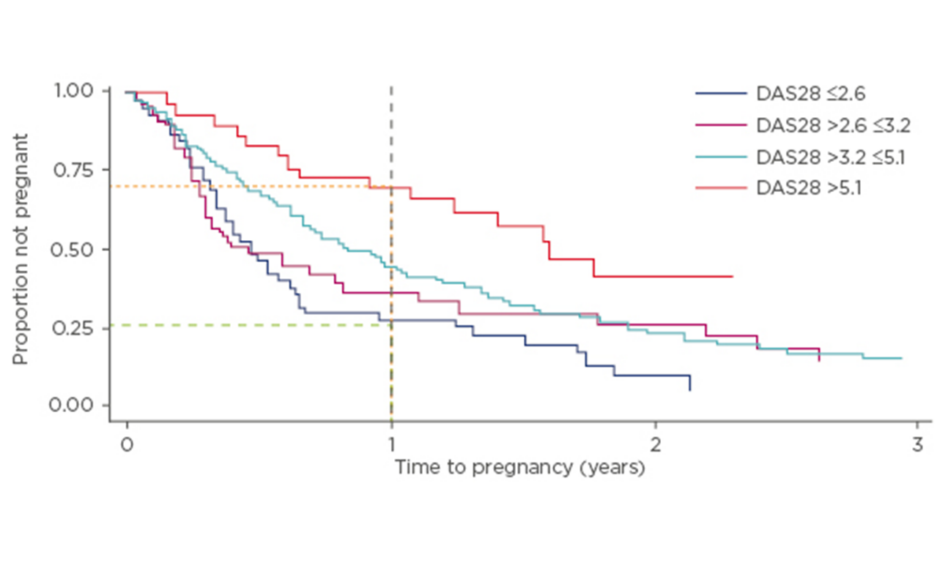
Since maternal age is one of the primary risk factors for infertility7 this is a concern in patients with chronic rheumatic diseases who are of childbearing age, particularly given the tendency to delay conception in this patient population. In a prospective study of 245 women with RA (American College of Rheumatology criteria) and a mean age of 31.3 years, time to pregnancy (TTP) was >12 months in 31% of patients, and 42% of women were considered to be sub-fertile relative to women without RA.8 In this cohort, only 15% of patients had received treatment with biologics, indicating representativeness of the general RA population. TTP was longer in patients who were older, nulliparous, and those with higher disease activity. For women with higher disease activity, for each point on the Disease Activity Score (DAS) 28 scale, there was a 20% reduction in the likelihood of pregnancy within the first 12 months (Figure 1). Approximately 75% of patients presenting with high disease activity did not achieve a pregnancy within the first 12 months, compared with 25% of patients with inactive disease.8
Figure 1: Higher DAS28 score results in a longer time to pregnancy.8
DAS28: Disease Activity Score 28 scale.
TTP was also delayed in women who had previously used nonsteroidal anti-inflammatory drugs (NSAID) or prednisone. Approximately 75% of patients taking pre-conception prednisone >7.5 mg/day failed to achieve pregnancy within 12 months.8 Although methotrexate (MTX) is teratogenic, available data suggest it has no impact on fertility.9 Limited data addressing the impact of anti-tumour necrosis factors (anti-TNF) on fertility in women without RA indicate that it is unlikely that fertility is decreased.10-12
For patients with chronic rheumatic conditions, a number of drugs (e.g., prednisone, methylprednisone, hydroxychloroquine) can be safely prescribed pre-pregnancy. Sulfasalazine should be prescribed in conjunction with 5 mg folic acid during the pre-conception period. However, it is recommended that MTX is discontinued at least 3 months prior to conception in women. For leflunomide, discontinuation prior to conception is strictly recommended with cholestyramine washout. Non anti-TNF biologics are not generally recommended during the peri-conception period; tocilizumab and rituximab should be discontinued ≥3 and ≥6 months, respectively, prior to conception.13
Optimising Outcomes for Mother and Baby During Pregnancy
Professor Catherine Nelson-Piercy
Disease Control Should Be Maintained Throughout Pregnancy
To achieve optimal pregnancy outcomes disease control before and during gestation is vitally important.14 The risk of drug therapy for the child should be weighed against the risk that untreated maternal disease represents for the woman and the fetus/child.6 Early initiation of a pre-pregnancy plan and continuity of care provided by a multidisciplinary team (i.e., rheumatologist, internist, obstetrician, gynaecologist, and other healthcare providers) is essential to support patients who have chronic rheumatic diseases.6,13,15 The treatment plan should be individualised according to the rheumatic condition, disease activity, symptoms, and the current treatment regimen. MTX should be withdrawn prior to pregnancy,6 since the teratogenic risk of MTX post-conception is 6.6% (versus around 3.0% risk in the general population), with an early miscarriage rate of 42% (versus 10–15% in the general population).16-18
Corticosteroids are generally considered to be safe during pregnancy; they are partially inactivated in the placenta and current evidence suggests that there is no overall risk for fetal malformation.19 However, patients should be counselled that short-term corticosteroid use increases the risk of gestational diabetes and infections, and prolonged use may be associated with maternal hypertension, diabetes, and osteoporosis, as well as an increased risk of pre-term delivery, pre-eclampsia, and fetal growth restriction.19 Corticosteroids should be administered with calcium and vitamin D for osteoporosis protection, along with a proton pump inhibitor.
Only 0.2% of pregnant women using immunosuppressive agents experienced a serious infection in a cohort study (N=4,961).20 Although the risk of serious infections increased during the later months of pregnancy, no statistically significant differences in serious infection risk were observed among pregnant women using non-biologics versus corticosteroids (hazard ratio [HR]: 0.81, 0.48–1.37), anti-TNF versus corticosteroids (HR: 0.91, 0.36–2.26), and anti-TNF versus non-biologics (HR: 1.36, 0.47–3.93). However, high-dose corticosteroid (>20–30 mg/day) use was an independent risk factor for serious infection in pregnancy (p=0.02).20
NSAID may be useful in axSpA, but should be discontinued where possible, particularly after 32 weeks.6,13 For anti-TNF therapy, EULAR and the British Society for Rheumatology (BSR) recommend continuation until a specified gestational week, with discontinuation recommended if the patient remains well and pain free.6,15 In the absence of clinical data for some anti-TNF, these guidelines state that consideration should be given to discontinuing anti-TNF therapy for the third trimester to limit neonatal exposure.
Treatment Should be Optimised to Prevent Flares
Recent evidence suggests that the use of anti-TNF therapy should be considered beyond conception to stabilise disease activity and prevent a flare during pregnancy in patients with RA or axSpA.21 Discontinuation of anti-TNF at the time of a positive pregnancy test was associated with disease aggravation in the second trimester in axSpA patients (n=24), with a relative risk of flare of 3.08 (95% confidence interval: 1.2–7.9).21 Importantly, following the re-introduction of anti-TNF or glucocorticoid treatment, disease activity remained elevated throughout pregnancy in 62.5% of patients.21
Treatment for Rheumatoid Arthritis or Axial Spondyloarthritis Does Not Preclude a Natural Birth
The presence of RA or axSpA is not a contraindication to vaginal birth, regional analgesia, or anaesthesia.
Biologics in Pregnancy
Transport of biologics across the placenta during pregnancy is not a class effect and may differ among the anti-TNF agents. Immunoglobulin-G1 subclass anti-TNFα biologics, infliximab and adalimumab, are actively transported across the placenta and the BSR recommend discontinuation in the second and third trimester, respectively.22,23 Similarly, etanercept cord blood levels are 3.6–7.4% of maternal levels and, as per BSR guidelines, should be discontinued in the third trimester.22,24 In contrast, active placental transport does not appear to occur with certolizumab pegol (CZP) (Figure 2), which may, as per the above guidelines, be continued throughout pregnancy.22 In the CZP placental transfer study, CZP levels were below the lower limit of quantification, using a highly sensitive CZP-specific assay, in 93% of infants at birth and 100% of infants at Weeks 4 and 8 (Figure 2).25 CZP post-marketing surveillance data up to 2014 report a live birth rate of 80.9%; of this 80.9%, the rate of congenital malformation was 4.3% and was not considered to be CZP-related.26 Limited data for golimumab preclude its recommendation before and during pregnancy.6
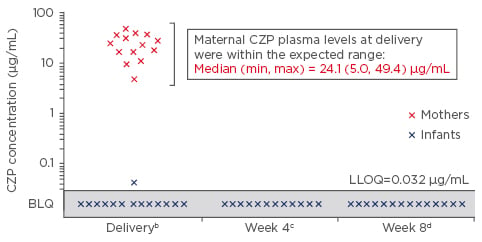
Figure 2: Certolizumab plasma concentrations in pregnant women recruited after 30 weeks’ gestation who received a dose of certolizumab within 35 days of delivery (n=14 mother–infant pairsa).
The primary outcome of this study was the neonatal blood level of CZP.25
a2/16 infant samples were excluded: 1 due to missing data at birth and 1 due to implausible PK data at birth (i.e., data not consistent with a paediatric CZP PK model based on the expected range of clearance, volume of distribution, and subsequent elimination half-life); b±24 hours; c±7 days (2 samples not collected); d±7 days.
BLQ: Below the lower limit of quantification; CZP: certolizumab; LLOQ: lower limit of quantification; PK: pharmacokinetics.
According to EULAR, continuation of anti-TNF during the first trimester should be considered; etanercept and CZP can be prescribed throughout pregnancy, because there is a lower risk of placental transfer (Grade B recommendation).6 The Delphi grade states that etanercept can be continued up to gestational Week 30-32 and, only if indicated, can be continued throughout pregnancy.6
Postpartum Flares and Breastfeeding
Doctor Megan Clowse
While there are limited data on the incidence of postpartum flares in axSpA, evidence suggests that postpartum disease activity worsens in up to 46% of women with RA or psoriatic arthritis.27,28 Many women with inflammatory arthritis wish to breastfeed and it is important that they hear from their physicians that most anti-rheumatic medications are considered safe with breastfeeding.
When considering treatment to prevent postpartum flares in a woman who is breastfeeding, it should be noted that medications do not collect in breastmilk like a reservoir, but instead the concentration correlates with the level of a drug in maternal blood. It peaks when the maternal drug levels peak and diffuses out of the breastmilk as the maternal level declines.29 The amount of drug that is actually transferred into breastmilk is dependent on two main factors: a) molecular size: very small molecules will diffuse freely into the breastmilk; and b) lipophilicity: lipophilic drugs will preferentially transfer into breastmilk.30 For a few drugs with higher levels of transfer, breastfeeding that is delayed by several hours post-dose may have lower levels of drug in the breastmilk.29 Experts recommend the acceptable relative infant dose of <10% of the maternal daily dose.30
The majority of medications that would be prescribed for chronic rheumatic diseases are considered compatible with pregnancy and lactation, including prednisone and ibuprofen.6,13,15 The prospective CRADLE CZP breastmilk transfer study sampled breastmilk at steady-state, using an optimised immune assay with increased sensitivity and specificity. It determined that one-third of women had low but measurable CZP in their breastmilk and that the mean average daily infant dose of CZP in breast milk (0.0035 mg/kg/day) is <1% of the expected therapeutic plasma concentration.3,31 The median infant dose of 0.15% (min, max: 0.04, 0.30) is considered unlikely to be of clinical concern.30 Infants of CZP-exposed mothers had a safety profile consistent with that of unexposed infants of a similar age.31 Of note, EULAR recommends that children exposed late in the second trimester or during the third trimester of pregnancy can follow vaccination programmes, but should not receive live vaccines in the first 6 months of life.6 Vaccines to avoid in the first 6 months include rotavirus and Bacillus Calmette–Guérin.17,32
In summary, pre-pregnancy planning and appropriate family planning are important for patients and partners in order to explain risks, overcome fears, and establish a treatment plan. Disease control before, during, and after pregnancy is important in order to achieve optimal maternal and fetal/child outcomes. A multidisciplinary approach with open and clear communication of the treatment guidelines is required to help with decision-making and to ensure patients are fully informed. Many drugs have an acceptable benefit–risk profile in pregnancy and breastfeeding.
Question and Answer Session
Q: Should we consider working in collaboration with a fertility specialist, and at what stage should the fertility specialist be brought into the process for women seeking to have a pregnancy?
A: Dr Moltó replied that clinicians should systematically ask the question to their patients regarding whether there is a wish to conceive. Generally, in young females, this is not done at every visit, and in young women of childbearing age it should be done, because if the question is not asked there may be an oversight with regard to problems with contraception and unplanned pregnancies with patients on MTX.
Q: Can you restart an anti-TNF agent postpartum after discontinuing due to pregnancy?
A: Prof Nelson-Piercy and Dr Clowse concurred that it is very important to reinitiate treatment with an anti-TNF agent as soon as possible postpartum. Disease flares may occur as early as 4 weeks post-pregnancy and, therefore, anti-TNF therapy should be restarted within 1–2 weeks after delivery.
Q: If a patient discontinued a class, such as an anti-interleukin-17 or an anti-interleukin-12/23, and the patient becomes pregnant, would you switch to another mechanism of action?
A: Dr Clowse responded that if a patient is taking a newer biologic, they should be prescribed an anti-TNF agent that they have not previously received (as treatment with previously-received anti-TNF agents typically fails), because many of the newer biologics do not have safety data to support their use during pregnancy.
Click here to view the symposium highlights.