Meeting Summary
Biosimilars have been available in Europe since 2006, and biosimilars of monoclonal antibodies since 2013, and are now a widespread clinical reality. Since their introduction, various sources of data have become available to help physicians make knowledgeable decisions about their use. For example, randomised clinical trials can demonstrate the comparable efficacy and safety between the biosimilar and its reference biologic. Real-world evidence from registries and individual clinical centres provide additional data on the actual use of biosimilars across different therapeutic indications and broader patient populations, including those who have switched from the reference biologic to the biosimilar, while offering additional understanding of the long-term safety and effectiveness. Well-informed decisions based on a solid understanding of these data are important and can help the physician guide the patient to their own well-informed decision, thereby reducing the possibility of a nocebo effect. Here we review available data sources and look at best practice examples of communicating with patients.
Introduction
Professor Chris Edwards
Over 20 biosimilar products are licensed in Europe, including two infliximab (INF) biosimilars (CT-P13 and SB2) and two etanercept (ETN) biosimilars (SB4 and GP2015). These biosimilars increase patient access to effective biologic treatment and offer opportunities for cost savings to healthcare organisations. For these benefits to be realised, healthcare professionals (HCPs) must be confident that biosimilars have similar efficacy and safety to their reference products. Furthermore, HCPs should consider how transferring this confidence in treatment to patients influences the likelihood of optimising adherence and limiting unwanted nocebo effects.
The different sources of information available to HCPs to gain that confidence and then use it to make informed shared treatment decisions with their patients were reviewed by international experts at a Biogen-sponsored interactive symposium at the European League Against Rheumatism (EULAR) 2017 congress. Additionally, the expert faculty and audience discussed the process, practicalities, and best practice when switching patients to biosimilar treatment. Prof Edwards commented: “[For me] we are thinking more broadly than biosimilars because there are messages and lessons within what we are going to talk about that influence how we treat patients, how we manage change, and how we work as individuals and departments.”
Randomised Clinical Trials: Laying the Foundation
Professor Chris Edwards
Prof Edwards stated: “There are different layers of information required to make a confident decision to change treatment, what do you need to know? We need to look at the Phase III studies, specifically the extension studies, to look at the switching of patients from one biologic to another.”
For novel biologics, the goal of the development process is to demonstrate de novo the risk-benefit profile of the candidate product, thus emphasising the role of clinical trials in this process. However, in biosimilar development, the reverse is true, as the aim is to demonstrate that the biosimilar is similar to the reference product, and to leverage the risk-benefit profile that has previously been established. Following comprehensive analytical comparability exercises and a Phase I pharmacokinetic comparability study, a Phase III randomised controlled trial (RCT) is conducted to demonstrate equivalent efficacy and comparable safety of the biosimilar with the reference product. Data from extension studies are available to demonstrate clinical outcomes following switching between reference products and biosimilars.
For example, the development programme for SB4 included a Phase III, 52-week, randomised, double-blind trial, where patients with moderate-to-severe rheumatoid arthritis (RA) despite methotrexate (MTX) therapy received either subcutaneous SB4 or ETN 50 mg every week. After 52 weeks of treatment, patients in the Czech Republic and Poland were enrolled into an open-label extension period for an additional 48 weeks and received SB4. Long-term safety and efficacy of SB4 were compared between patients who continued SB4 (SB4/SB4; n=126) and those who switched from ETN to SB4 (ETN/SB4; n=119).1 The mean disease activity score based on 28 joints and simplified disease activity index were found to be comparable between the two groups during the extension period, suggesting efficacy is sustained after switching from ETN to SB4. Switching from ETN to SB4 also resulted in no treatment emergent issues, suggesting SB4 was well-tolerated and effective over 2 years in patients with RA.2
“Overlap of confidence intervals suggests there is no evidence of difference in disease activity scores achieved with these agents. Safety, being of great importance whenever starting a new drug, was also similar between the two groups regardless of whether they continued on SB4 treatment or switched from ETN to SB4,” noted Prof Edwards.
Another Phase III study investigated the safety and sustained efficacy in patients with moderate-to-severe RA who were randomised to receive either SB2 or INF until Week 46. At Week 54, patients previously receiving INF were re-randomised to either receive SB2 (INF/SB2; n=94) or continue INF (INF/INF; n=101) up to Week 70; those patients initially receiving SB2 continued to receive SB2 (SB2/SB2; n=201).3 Efficacy, safety, and immunogenicity were comparable between the three treatment groups up to Week 78, suggesting SB2 was well-tolerated and effective even in patients who switched from INF to SB2.
Cross-Reactivity
Additional analysis to confirm cross-reactivity between the biosimilar and reference product can provide further support of the similarity of these agents and provide additional confidence when considering switching patients. For example, a recent study aimed to determine whether antibodies to INF (ATI) developed in patients with inflammatory bowel disease treated with INF or CT-P13 or, having switched from INF to CT-P13, cross-reacted with the biosimilars CT-P13 and SB2. Three bridging enzyme-linked immunosorbent assays were used to measure ATI levels and results suggested that similar immunodominant epitopes on the reference and biosimilar drugs are responsible for the same degree of reactivity, and that the Promonitor®-ANTI-INF (Grifols, Barcelona, Spain) test can be used to detect antibodies to CT-P13 or SB2 in patients treated with biosimilars.4
“Thinking about immunogenicity, does switching patients between reference product and biosimilars mean these monoclonal antibodies are similarly recognised by the immune system in these patients? Do the anti-drug antibodies bind to similar, immune epitopes, of these drugs? The same levels of anti-drug antibodies were seen in these patient groups, suggesting antibodies against one type of INF antibodies will bind to other INF-type molecules in the form of CT-P13 and SB2 as well,” mused Prof Edwards.
Registry Data: Framing the Knowledge-Base
Professor Merete Lund Hetland
Additional evidence that complements RCT data is derived from registries. DANBIO, a mandatory nationwide registry in Denmark, is capturing routine practice across the country. In 2015, according to guidelines, a switch for non-medical reasons (non-medical switch) from INF to CT-P13 was implemented in Denmark. Following the approval of SB4 in 2016, patients receiving ETN also experienced a non-medical switch to SB4.
Prof Hetland explained: “In Denmark, the originators and biosimilars are treated as equals by the authorities. The HCPs know what the patient is receiving, whether it’s an originator or a biosimilar, and can therefore monitor their response to the individual treatments in DANBIO, enabling us to study effectiveness of the biosimilar drugs.”
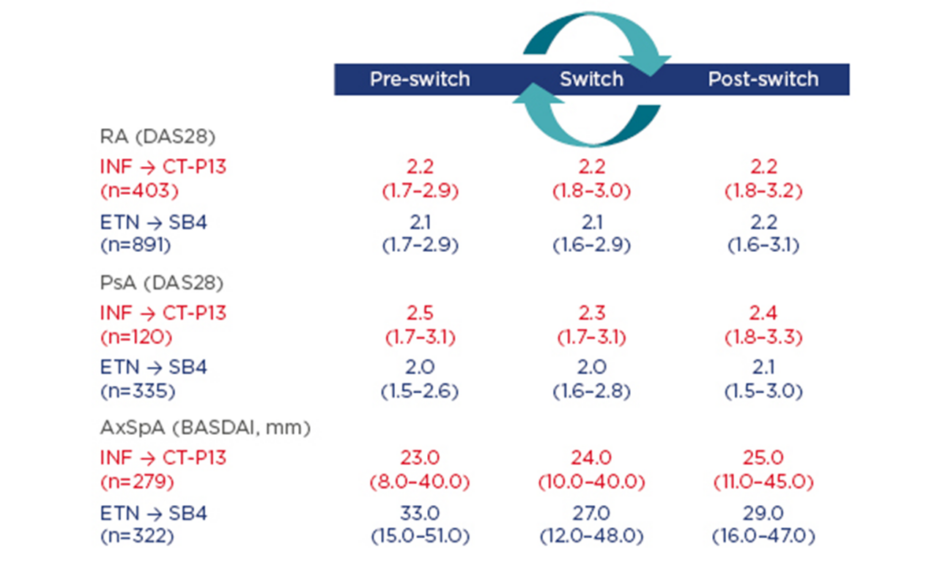
Using data from DANBIO, the following observational study aimed to investigate the impact of a nationwide switch from INF to CT-P13 and from ETN to SB4 on disease activity and flare rates 3 months before, during, and 3 months after switching patients to CT-P13 or SB4. In patients treated with CT-P13, reasons for withdrawal were analysed and the CT-P13 retention rate was compared with an historic cohort of INF-treated patients.5,6 Patients who switched from INF to CT-P13 had prior INF treatment duration of 6.3–7.3 years. Results showed no evidence of clinically relevant differences in disease activity across indications (RA, psoriatic arthritis [PsA], and axial spondyloarthritis) (Figure 1) and similar flare rates were seen pre and post-switch.
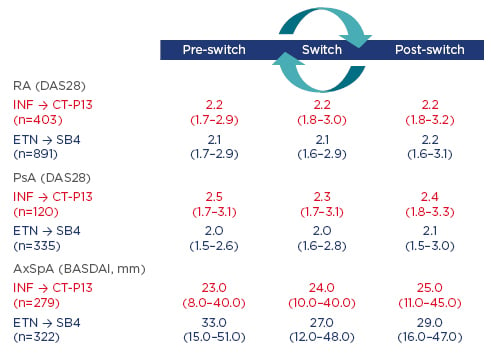
Figure 1: Disease activity 3 months prior to versus 3 months after the switch stratified by diagnosis.
AxSpA: axial spondyloarthritis; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; DAS28: disease activity score in 28 joints; ETN: etanercept; INF: infliximab; PsA: psoriatic arthritis; RA: rheumatoid arthritis.
Of the 132 patients (16%) who discontinued CT-P13 treatment within a year, lack of efficacy and adverse events (AEs) were registered as reasons for withdrawal in 71 and 37 patients, respectively. As all patients were switched to biosimilar treatment, there was no control group to compare retention rates against and, therefore, historic cohort group data were used. This adjusted analysis (Multivariable Cox regression analysis adjusted for the following baseline variables: age, sex, diagnosis, MTX treatment [yes/no], comorbidities [number], and patients’ global score) found the absolute retention rate of CT-P13 was significantly different by 3.4% from that of INF in the comparison cohort, suggesting CT-P13-treated patients had a slightly higher risk of withdrawal than INF-treated patients.
Patients who switched from ETN to SB4 had received prior ETN treatment for an average of 5.2 years, and a large proportion of patients with RA (60%) and PsA (49%) received concomitant MTX. In this population, 3-month disease activity and flare rates were largely unaffected by switching patients to SB4 (Figure 1). Discontinuation of SB4 (n=129; 9%), during 5 months treatment, was mainly reported as lack of effect or AEs. A higher patient global score (hazard ratio [HR]: 1.12/cm; 95% confidence interval [CI]: 1.05–1.21; p=0.002) and no concomitant MTX (HR: 2.28; 95% CI: 1.48–3.52; p<0.001) at baseline were associated with higher discontinuation rates.
These observational nationwide studies of 2,350 patients with RA, PsA, or axial spondyloarthritis who undertook a non-medical switch to biosimilars found disease activity and flare rates were largely unaffected. Biologic-naïve patients are also being prescribed biosimilars in Denmark due to national guidelines recommending the use of the most economical options for a biologic.
Patient Perspective: Transfer of Knowledge
Associate Professor Lars Erik Kristensen
Assoc Prof Kristensen quoted Bernard Lown: “Words are the most powerful tool a doctor possesses, but words, like a two-edged sword, can maim as well as heal.”
Although RCT and real-world data detailing the efficacy/effectiveness and safety of a medication are important when making treatment decisions, HCP interaction with their patients, and how it fundamentally influences the likelihood of success or failure of a treatment, is also vitally important. A recent example of how communication can strongly influence treatment outcomes was published.7 In this study, both patients and physicians were blinded to treatment; patients were randomised to receive either statin or placebo. During the double-blind phase, statin-treated patients reported similar AEs to that of the placebo group (298 in the treatment group versus 283 in the placebo group; HR: 1.03; 95% CI: 0.88–1.21; p=0.72). However, a significant excess in muscle-related AEs was reported during the open-label phase when patients and physicians both knew statin treatment was being administered (161 versus 124; HR: 1.41; 95% CI: 1.10–1.79; p=0.006). This was reported to be due to a nocebo effect, the negative equivalent to a placebo effect that can lead to the induction or the worsening of symptoms, which likely resulted from negative perceptions about statin use or due to poor understanding of statin side effects. This highlights the importance patient perspective plays in promoting patient empowerment and adherence to treatment, which leads to optimal disease management.
The likelihood of a nocebo effect is influenced by different factors, including patient-related factors (e.g. psychiatric illness or personality traits), verbal and non-verbal communication, which may unintentionally contain negative suggestions or psychological factors (negative expectations and suggestibility), and neurobiological factors. Research at the Parker Institute, Copenhagen University Hospital, Bispebjerg and Frederiksberg, Copenhagen, Denmark, is advancing this field by qualitatively exploring disease and treatment- related issues and concerns experienced by patients with RA, PsA, and psoriasis, and trying to understand the clinical importance of these concerns.8 The Parker model consisted of three stages: 1) concept mapping, a structured focus group process, which was used to identify and organise disease and treatment-related issues and concerns. Data were organised using participants’ themes, multidimensional scaling, cluster analysis, participant validation, rating of clinical importance, and thematic analyses, to generate a conceptual model of disease-related concerns experienced by patients (Figure 2); 2) participatory design, which consisted of consecutive iterative sessions with four patients to achieve four final prototypes that answered the question: “How would your ideal communication work and which elements would you need?”; and 3) stakeholder evaluation, which consisted of individual and group interviews and was carried out to explore the applicability and relevance of introducing these findings into the clinic.
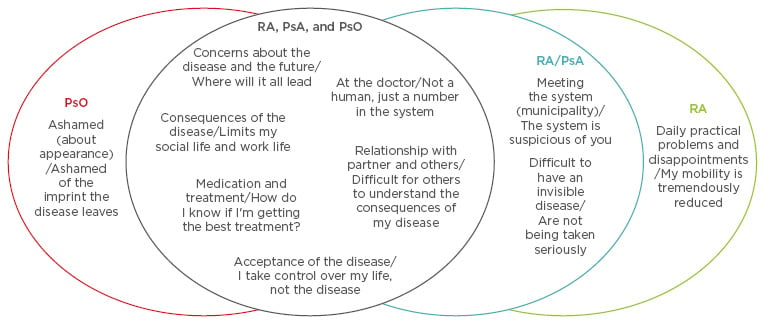
Figure 2: Disease-specific concepts generated during patient workshops.
PsA: psoriatic arthritis; PsO: psoriasis; RA: rheumatoid arthritis.
Jørgensen TS et al. Identifying Generic and Specific Patients’ Perspectives on Disease and Treatment Related Issues in Rheumatoid Arthritis, Psoriatic Arthritis, and Psoriasis: A Qualitative Concept Mapping Study. Arthritis Rheum. 2016;68(Suppl. S10):Abstract 1422. Copyright © 2016. Reproduced with permission of John Wiley & Sons, Inc.
Results from this study suggest a three-step approach should be taken when switching patients from a reference biologic to a biosimilar: 1) patients should be informed about the switch well in advance; 2) an appointment with the physician should ideally take place to allow time with the patient to gauge their level of anxiety regarding the switch; 3) a clinical visit to administer the drug under supervision should occur. The chosen wording (i.e. biosimilar/copy/generic/ identical) is sensitive and important for patient understanding and empowerment when informing about switching. These qualitative data highlight the importance patient–physician communication has in RA, PsA, and psoriasis. Although the nocebo effect can impair drug performance, effective patient–physician interaction can minimise this.
“This highlights the importance of how physicians communicate with patients, how topics are discussed and the information provided to the patient: balancing the positive and the negative information correctly. This way of communicating may be very important when switching a patient from one drug to another,” stated Prof Edwards.
Implementing Change: Real-Life Experiences of Introducing Biosimilars to Patients
Professor Chris Edwards and Doctor Maria Cuadrado
Transitioning patients from a reference biologic to a biosimilar is an important topic, partly due to cost savings. Since this is now a clinical reality, practical guidance on step-by-step implementation of switching is increasingly sought and can be gained most effectively by sharing best practice from institutions that have already made the change. For example, in the UK, two large teaching hospitals, University Hospital Southampton, Southampton and Guy’s and St Thomas’, London, have switched patients from reference biologics to biosimilars and reported outcomes.9-11
At the University Hospital Southampton, all patients with rheumatic diseases were switched from ETN to SB4. Previous involvement in biosimilar trials, working with colleagues with experience of switching patients in other indications, and previous experience of switching patients from INF to CT-P13 provided physicians with the confidence to switch all their patients simultaneously.9,10
The strategy that was implemented focussed on educating both patients and colleagues regarding biosimilar development, treatment, and recent advances. All stakeholders had to agree on the approach taken and if >10% of patients failed on the biosimilar they agreed to stop switching. Patients were assured that they could switch back to ETN if they were unhappy for any reason. Information provided to patients included a letter accompanied with an information sheet detailing the switch, followed by a face-to-face discussion with a consultant rheumatologist and specialist rheumatology nurse. A telephone helpline was also available, which was operated by rheumatology nurses, to provide support to patients and allow them to make an appointment with a rheumatologist and report any AEs. A routine clinical review appointment was also arranged at 3 months after switching and then 6-monthly thereafter or more frequently if required. Following the education and support programme, 99% of patients (n=92) with RA, PsA, and AS, agreed to switch from ETN to SB4. Six months after the switch, 91% of patients had continued treatment with SB4 with sustained efficacy outcomes. Those patients (n=8) who discontinued treatment reported inefficacy or AEs, and it should be noted that these discontinuation rates were comparable to those seen with ETN in the control period 6 months prior to switch. “In the process we went through to make change, we made sure we were giving as much information as possible to patients, we discussed in advance what we were going to do as well,” noted Prof Edwards. From this experience, it is clear that informed patient choice, education, and agreement from all stakeholders is vital to effectively switch therapies. It is also important that patients do not feel under pressure and are given the option to revert to previous therapy.11
A similar approach to switching patients to biosimilars was taken at Guy’s and St Thomas’ Foundation Trust (Figure 3). A multidisciplinary team consisting of managers, rheumatologists, and pharmacists discussed the option to switch patients from their reference biologic to a biosimilar. After extensive research and in-depth analysis of potential cost savings and the positive impact this could have, the decision was taken to switch all patients from ETN to SB4 and also prescribe SB4 to biologic-naïve patients. “We have a fixed budget for drugs, so if we save money we can treat more patients with biologics and can also treat other patients with other drugs that are expensive where we have limitations. So, the money saved will go back into the system to treat other patients,” commented Dr Cuadrado.

Figure 3: Process and informative letter used to switch patients from ETN to SB4.
ETN: etanercept; HCP: healthcare professional.
A key feature of the letter was that it was signed by every member of the multidisciplinary team to highlight to the patient that this was an informed shared decision and therefore provide the patient with confidence. After receiving the letter, only 5 out of 112 patients requested to speak with their physician before consenting to switching. From April 2016–October 2016, 112 patients were switched from ETN to SB4 and 110 biologicnaïve patients were successfully initiated on SB4. “Sometimes we are rushing, so we have to implement a system that does not disrupt us and saving money can help provide extra resources to do that. If patients, after reading the letter, wanted to speak with somebody, they could go to the clinic and ask [the specialist nurse or pharmacist] whatever they wanted,” declared Dr Cuadrado.
Conclusions
Dr Cuadrado concluded: “When we started using anti-tumour necrosis factors, we did not know what was going to happen, we did not have long-term data, but we used it and continue to use it despite safety concerns we had/have, this is because the risks do not overweigh the benefits and we have seen the lives of our patients change with anti-tumour necrosis factors. So, I now think the same approach should be taken with biosimilars.”
Given the positive impact biosimilars can have on patients, individual departments, and healthcare systems as a whole, it is important that HCPs have access to information from multiple sources to develop the confidence to successfully implement their use. Additionally, a well-informed HCP is better placed to transfer confidence to patients and reduce the possibility of a nocebo effect.
“How do you get information to make you comfortable about the treatments you use in all different situations? We usually look at the RCTs, we look at the information presented at meetings like this, then we look at real-world registry data, and we learn from our own experience in our clinical centres where all these layers of information build up the pieces of a jigsaw… we keep testing and questioning all the time and remain slightly uncertain until we have a long duration of experience with any medication,” said Prof Edwards.