Meeting Summary
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) causes irreversible short- and long-term damage to vital organs, particularly the kidneys and lungs. Current standard of care (SOC) for AAV, of which glucocorticoids (GC) are a lynchpin, has a number of important limitations: responses to therapy are variable, some patients fail to achieve and sustain remission, and treatment related adverse events (AE) are common. GCs in particular carry a heavy toxicity burden leading to increased organ damage and other serious AEs, as well as negatively impacting patients’ quality of life. During this symposium, leading vasculitis experts considered how to strike the right balance in AAV and achieve the dual clinical priorities of sustained disease control and minimisation of treatment-related toxicity. The emerging evidence for alternative complement pathway activation in AAV disease pathogenesis and its investigation as a potential therapeutic target were also discussed, with promising results from the landmark Phase III ADVOCATE trial having recently demonstrated the non-inferiority of the novel C5a-inhibitor avacopan versus SOC at Week 26 and the superiority at Week 52.
The Pathogenesis of Vascular Lesions and the Alternative Complement Pathway
Benjamin Terrier
AAV are a type of small-vessel necrotising vasculitis that manifest as systemic diseases, with pulmonary; ear, nose, and throat; and renal involvement. The key pathological feature of AAV is the presence of ANCA, which can target two neutrophil antigens, myeloperoxidase (MPO) and proteinase 3 (PR3). Granulomatosis with polyangiitis is more commonly associated with PR3, while microscopic polyangiitis and eosinophilic granulomatosis with polyangiitis (previously known as Churg-Strauss) are typically associated with the MPO serotype.
Terrier discussed how the pathogenic role of MPO-ANCA was first demonstrated two decades ago. Treatment of mice with MPO-ANCA purified from MPO-deficient mice or via transfer of splenocytes into immunodeficient mice was shown to induce a glomerular nephritic disease after 6 days. The glomeruli of these mice exhibited a fibrinoid necrosis with crescents with very few IgG deposits, indicating a pauci-immune glomerular nephritis as seen in the human disease.1
Further work then set out to explore the role of neutrophils in disease pathology. An important genome-wide association study demonstrated that there is a distinct genetic background according to ANCA specificity, with PR3 ANCA patients, but not MPO-ANCA patients, showing polymorphisms of key genes encoding α-1 antitrypsin and PR3.2 Neutrophils have also been established as a key effector of endothelial damage in AAV. Analysis of the characteristics of neutrophils in patients with AAV revealed specific gene expression profiles, specifically disturbed epigenetic control of PR3/MPO expression.3-5In vitro data demonstrated that MPO and PR3 are able to induce a proinflammatory environment in endothelial cells, including the production of proinflammatory cytokines, while in vivo experiments have shown that ANCA is able to stabilise the endothelial adhesion of neutrophils and increase their transmigration, thereby driving inflammation.6-10
The neutrophil antigen PR3 has also been proven to play a key role in the inflammatory process that underpins AAV. Patients with AAV show constitutive expression of membrane PR3 (mPR3), which acts as a danger signal for macrophages and disrupts immune signalling. Apoptotic neutrophils expressing mPR3 induce a proinflammatory response by macrophages, which then induces maturation and activation of dendritic cells, creating a favourable microenvironment for the persistence of inflammation.11-13
The complement pathway has emerged as another potential culprit with a role in AAV pathophysiology. In a murine model of MPO-ANCA vasculitis induced by intravenous injection of MPO-ANCA, mice pre-treated with cobra venom factor (a toxin that depletes complement) did not develop renal disease.14 This clearly illustrates the critical importance of the complement pathway in disease initiation, noted Terrier. Further work in mice with a different genetic background showed that those missing genes from the alternative complement pathway, particularly C5 or factor B, were protected from developing vasculitis in the kidney, suggesting that ANCA-stimulated neutrophils release factors, which activate complement via the alternative pathway.14 Mice with deleted C5a pathway genes, particularly those coding for the C5a receptor, were similarly protected from MPO-ANCA glomerular nephritis and animals treated with CCX168, a molecule that specifically inhibits the C5a receptor, developed less severe forms of the disease as evidenced by decreased haematuria, proteinuria, and leukocyturia.15
Research into AAV also has probed the interaction between activated neutrophils and factor H. TNF-primed neutrophils exhibit a strong inflammatory reaction when bound to endothelial cells, including respiratory burst and degranulation. Binding factor H to these neutrophils acts to inhibit ANCA-induced neutrophil activation. However, in patients with AAV, factor H displays a deficient ability to bind to neutrophils and inhibit their ANCA-induced activation.16
Integrating all of these different mechanistic aspects gives a clear insight into the interconnected role of ANCA, neutrophils, and complement in AAV.17 Activation of neutrophils by cytokines or C5a induces membrane expression of MPO/PR3 antigens. These primed neutrophils then undergo activation by ANCA, inducing an oxidative burst and endothelial damage. Activated neutrophils in turn release factors that activate the alternative complement pathway, leading to production of C5a and further amplification of the inflammatory response. The result is a positive inflammatory feedback loop.17 Terrier noted that, from a therapeutic perspective, targeting activation of the alternative complement pathway, in particular the key C5a/C5a receptor interaction, could be clinically beneficial and is one of the primary approaches currently under evaluation.
Overall, Terrier concluded that emerging evidence from murine models, human correlation studies, and data from randomised controlled trials support a key role for the alternative complement pathway in AAV. There are also strong interactions between neutrophil activation and their ability to activate the complement pathway, creating the possibility of combination therapies that target both. Potential therapeutic targets within the complement pathway include blockade of complement activation by C5a/C5aR inhibitors, which is emerging as a very promising strategy, noted Terrier. Other future complement-targeting approaches may include restoration of the regulator function of factor H.
Glucocorticoids in ANCA-Associated Vasculitis: Clinical Efficacy and Short-Term Risks. Where Do We Stand?
Joanna Robson
GCs are recommended at every stage of the European Alliance of Associations for Rheumatology (EULAR) management pathway for AAV as induction therapy, treatment for organ or life-threatening disease, as maintenance agents, and to manage relapse.18 GCs therefore represent a constant in patients’ lives, explained Robson, and exert a direct impact in terms of outcomes, AEs, and quality of life.
From a historical perspective, mortality remained high when GCs were the only available treatments for AAV.19 Today, the increased risk of infection associated with high-dose GCs remains a key concern for clinicians and is underscored by data from observational studies. A Japanese study showed that patients on high-dose GCs had a 50% cumulative incidence of severe infections over the course of 1 year.20 In another retrospective, multicentre study (n=114) from Europe and the USA, intravenous methylprednisolone significantly increased the risk of infection during the first 3 months (hazard ratio: 2.7; 95% confidence interval: 1.4–5.3), even after adjustment for confounding factors.21 Evidence from this same study also showed no benefit of methylprednisolone pulses versus standard-dose GC therapy in terms of survival, renal recovery, or relapses.21
Robson showcased data from a recent systematic literature review of 91 studies (published between 2000 and 2020), which included 18 randomised controlled trials and encompassed over 10,000 patients with AAV, highlighting the severe AEs associated with GC use. Infections were found to be the overwhelming risk, noted Robson, but other severe AEs were also of concern such as gastrointestinal haemorrhage or ulcers and musculoskeletal disorders.
In long-term follow-up of six randomised European Vasculitis Society (EUVAS) trials (n=302), almost one-half (48%) of patients were still receiving GCs after 7.3 years from diagnosis, which reflects the reliance on these drugs in clinical practice.22 Damage related to active vasculitis and its treatment were also shown to increase over time, with several of the most frequent Vasculitis Damage Index (VDI) items attributable specifically to steroids.22
Turning to patients’ perspective of steroids, Robson explained her involvement in a multinational project to develop a robust and well-validated patient-reported outcome (PRO) measure for AAV.23 The resulting AAV-PRO contains six different domains: organ-specific symptoms, systemic symptoms, treatment side effects, social and emotional impact, concerns about the future, and physical function; all of which individually fit the Rasch model and show good internal consistency.23 Specifically, Rasch analysis revealed treatment-related side effects as being of great importance to patients (p=0.065), with the underlying constructs (e.g., concern about appearance, weight gain, sleep problems, gastrointestinal complaints, and skin issues) all related directly to steroids.23 A secondary analysis of the quantitative data from this study specifically explored patient perceptions of GC therapy in AAV.25 Results showed a complex pattern across the patient journey.24 As Robson explained, at diagnosis patients often feel relief or gratitude when starting on GCs, which exert a swift effect on their symptoms. However, short-term AEs are common experiences that quickly lead to patients developing fears about the potential long-term impact of GCs. This can be fuelled by the negative connotations around steroid use from family, friends, and wider society. As AAV enters the chronic stage, the ‘weighing up’ process of GC benefits versus risks becomes even more pronounced and can impose a substantial cognitive burden on patients. Patients come to depend on steroids for their health, explained Robson, so must balance fears around ongoing and future GC AEs against the fear of a potential disease flare-up if steroid doses are reduced.
An earlier study into the burden of disease in AAV also identified treatment-related aspects as of key importance to patients.25 Frequent burdens identified in this study, such as fatigue or energy loss, weight gain, financial or work issues, and anxiety, could be linked directly to steroid use.25 Looking at health-related quality of life (HrQoL) in AAV, symptoms of depression and anxiety have been found to be highly prevalent, affecting 25.5% and 42.3% of patients, respectively.26 In qualitative surveys, patients described high-dose GCs as a direct contributor to this adverse HrQoL, with positive improvements seen as doses were reduced. The association of specific factors with work disability among working-age patients with AAV has also been explored in a cross-sectional study (n=208). Independent predictors were fatigue (odds ratio [OR]: 7.1), which can be linked to poor sleep caused by GCs, being overweight (OR: 3.4), and depression (OR: 4.4).27
The OMERACT Glucocorticoid Working Group is an international consensus body that is developing a core outcome set for future clinical trials involving GCs. Domains include hard clinical end-points that can be well-measured using the GC toxicity index (e.g., hypertension and diabetes) plus more patient-focused outcomes such as appearance, sleep disturbance, and mood disturbance.28 Robson explained that the Working Group have identified a critical gap in this area as currently there is no instrument available to accurately categorise these PROs and measure them effectively in a clinical trial setting. To plug this gap, work is underway to develop a steroid PRO capable of capturing patient perspectives on GCs for rheumatic diseases. This project will consist of initial qualitative interviews with over 50 patients from the UK, USA, and Australia, followed by a larger-scale survey to determine final scale structure and measurement properties/validation.
In summary, although GCs constitute effective treatments for AAV, they are associated with a range of short- and long-term AEs. Consequently, there is an impact on HrQoL, not only from AAV disease itself but also from treatment. It is important to consider and measure this impact of GCs from both physician and patient perspectives, Robson concluded.
Getting the Balance Right: Emerging Therapies in ANCA-Associated Vasculitis
Bernhard Hellmich
AAV is a rare disease, but recent data indicate that both prevalence and incidence are increasing. Based on retrospective, longitudinal analysis of health insurance data from >3 million Germans, the prevalence of granulomatosis with polyangiitis and microscopic polyangiitis currently stands at 256 per 1 million, with an annual incidence of 46 per 1 million.29
Hellmich outlined evidence for the outcomes obtained with current induction therapies recommended in EULAR AAV management guidelines.18 Long-term survival data from four randomised EUVAS trials showed that mortality in patients treated with standard therapy was high (OR: 2.6), with the main causes of death in the first year being uncontrolled vasculitis (19%) and infections (48%).30,31 The question of whether outcomes could be improved by plasma exchange or GC sparing were addressed in the PEXIVAS trial, the largest study ever conducted in AAV, which enrolled 704 patients with severe disease followed for up to 7 years.32 Plasma exchange had no significant impact on the primary composite end-point of death and end-stage renal disease at any timepoint in the study. However, reduced-dose GC proved non-inferior to standard-dose/taper over the whole study period and was associated with a significantly lower risk of serious infections in the first year (27% versus 33%).32
“If lower-dose GCs work, then can patients potentially be managed with no steroids at all?” asked Hellmich. This challenging hypothesis was recently addressed in two studies investigating the C5a receptor antagonist avacopan. The CLEAR, Phase II, proof-of-concept study enrolled 67 patients with active AAV and renal involvement who were assigned to one of three arms: standard therapy with high-dose GC, low-dose GC (20 mg starting dose) and avacopan, or avacopan and no GC.33 The primary end-point of ≥50-point reduction in Birmingham Vasculitis Activity Score (BVAS) at Week 12 and no worsening in any body system was achieved in a similar proportion of patients in all three arms: 70.0%, 86.4%, and 81.0%, respectively. Disease activity, as measured by percent change in BVAS, decreased rapidly, with a faster decline seen in the avacopan groups as compared to the steroid-only arm.33
Promising results from this proof-of-concept study led to the design and conduct of the larger Phase III ADVOCATE trial of avacopan, the results of which were recently published in the New England Journal of Medicine.34,35 ADVOCATE enrolled 300 patients with active AAV (either new disease or relapsing), who received standard induction therapy and were randomised 1:1 to avacopan 30 mg twice daily for 1 year plus placebo matching prednisone, or placebo plus prednisone starting at 60 mg/day and tapered to zero for 20 weeks. Patients were stratified according to baseline therapy (oral/intravenous cyclophosphamide or rituximab), ANCA type (anti-MPO or anti-PR3), and new or relapsing disease.35 Overall, patients were well-balanced across study arms: mean BVAS score was approximately 16, indicating severe disease, and most patients had impaired renal function (mean estimated glomerular filtration rate [eGFR] of approximately 50 mL/min/1.73m2).35
The dual primary end-points of ADVOCATE were remission at Week 26 and Week 52. In the avacopan group, 72.3% of patients achieved remission at Week 26 compared with 70.1% in the control SOC arm, confirming the non-inferiority (p<0.0001) of the avacopan-based regimen to GCs. At Week 52, the avacopan-based regimen proved superior to GCs, with 65.7% of avacopan-treated patients remaining in remission versus 54.9% on SOC; this difference was statistically significant (p=0.0066) (Figure 1).35
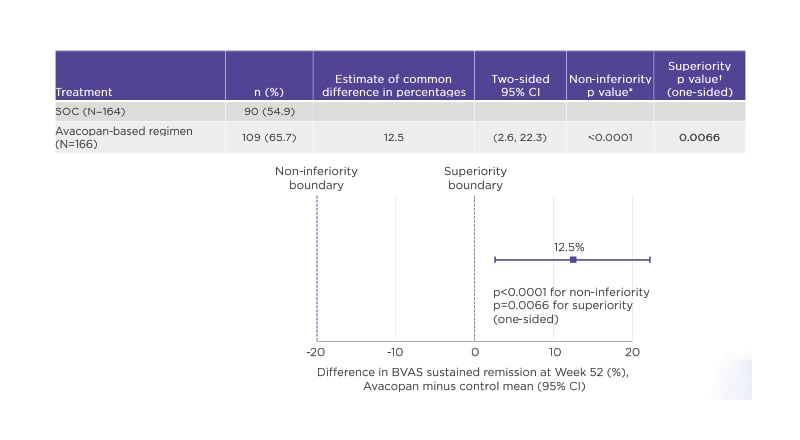
Figure 1: Avacopan was superior to glucocorticoids in sustaining remission at Week 52 (ADVOCATE primary end-point 2).36
*Boundary for non-inferiority <20%.
†Boundary for superiority <0%.
BVAS: Birmingham Vasculitis Activity Score (BVAS); CI: confidence interval; SOC: standard of care.
As would be expected with more patients sustaining remission, the relapse rate was also significantly lower in the avacopan arm versus SOC. Relapse rate was 10.1% with the avacopan-based regimen compared with 21.0% with SOC (p=0.0091), equivalent to a 54.0% reduction in relative relapse risk.35 Significant improvements in renal function were also seen with the avacopan-based regimen. In approximately 80% of patients with renal disease at baseline, avacopan produced a better recovery of renal function compared to SOC, with the benefit particularly marked in those patients at the lowest tertile of the eGFR.35 Deterioration in renal function is an indicator of worse prognosis in AAV, noted Hellmich.
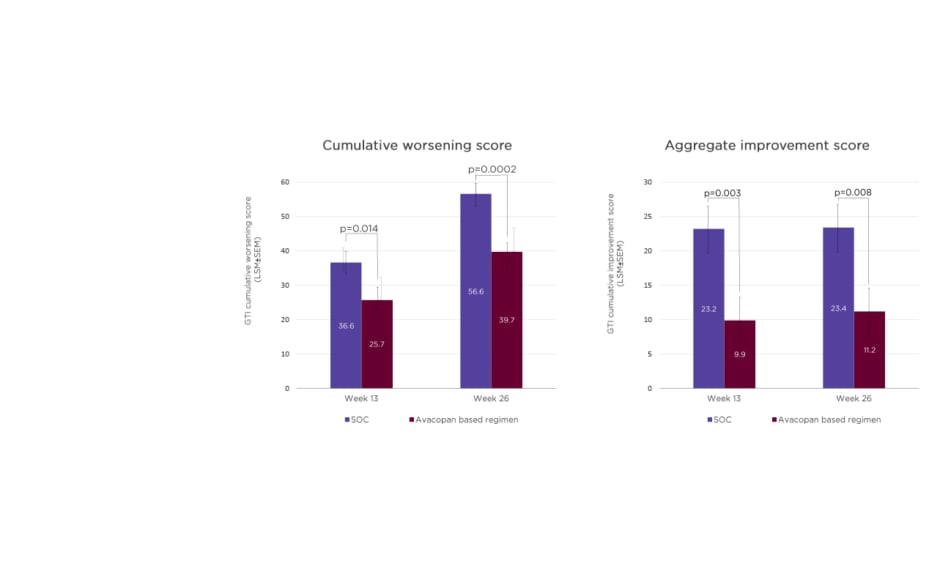
In the ADVOCATE study, the mean total prednisone-equivalent dose of GCs was higher in the SOC group (3,655 mg) compared to the avacopan-based regimen (1,349 mg). However, the avacopan group was not GC-free, Hellmich pointed out, with additional sources including pre-randomisation GCs, which were then tapered, co-medication with rituximab, and off-protocol doses.35 At both Weeks 13 and 26, avacopan-treated patients had a significantly lower cumulative burden of GC toxicity, as measured by the glucocorticoid toxicity index, versus SOC. A significant difference in toxicity between avacopan and SOC was also evident in aggregate improvement scores on the glucocorticoid toxicity index at Weeks 13 and 26 (Figure 2).35
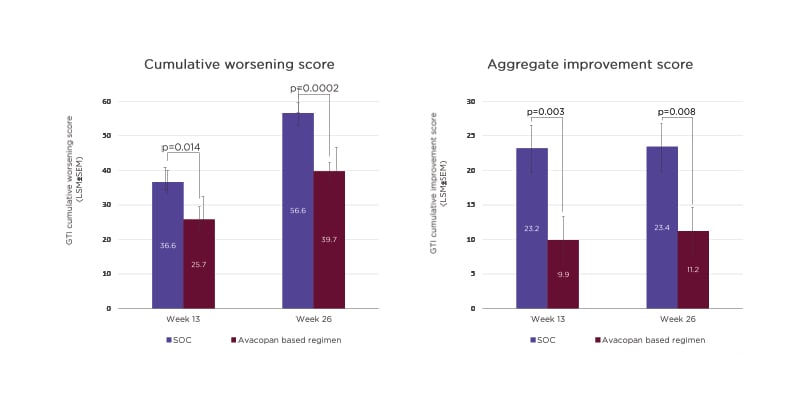
Figure 2: Glucocorticoid toxicity index scores in the ADVOCATE trial.36
GC: glucocorticoid; GTI: glucocorticoid toxicity index; LSM: least squares mean; SEM: standard error of the mean; SOC: standard of care.
Overall, the improved GC-related toxicity profile with avacopan translated into a numerically lower rate of AEs compared to the SOC group; however, severe AEs occurred at a similar rate in both arms (23.5% versus 25.0%, respectively). Severe infections were also numerically lower in the avacopan group, but this did not significantly impact the number of deaths. Total GC-associated AEs occurred more frequently in the SOC group (80.5%) as compared to avacopan (66.3%).35
Hellmich then turned to the evidence supporting drugs for maintenance of remission in AAV. In the MAINRITSAN trial, rituximab (500 mg every 6 months) proved superior to azathioprine (tapered after 12 months), and was associated with a lower rate of relapse at Month 28.36 More recent data from the RITAZAREM trial, which employed a higher dose of rituximab (1,000 mg every 4 months)versus azathioprine for 2 years, confirmed this significantly lower relapse risk with rituximab versus azathioprine.37 Only 13% of rituximab patients experienced relapse by Month 24, compared with 38% on azathioprine. Rituximab was superior in preventing both minor and major relapses, and relapse risk was not influenced by ANCA type, relapse severity, or GC induction. No new safety signals emerged in the RITAZAREM trial.
“If rituximab is such an effective drug, should it really be stopped after 2 years as per EULAR recommendations?” questioned Hellmich. The placebo-controlled, extension study MAINRITSAN-3 set out to answer this question by randomising 97 patients to either stop rituximab after 18 months or continue treatment for an additional 18 months.38 Patients on long-term rituximab showed a significantly lower relapse risk versus the control, with relapse-free survival rates at Month 54 (the primary end-point) of 96.0% versus 74.3%, respectively. After 36 months, rituximab therapy also proved 100.0% effective in preventing severe relapse to Month 54.
No new safety signs occurred with long-term use of rituximab, and severe AEs (including infections) were similar across both arms.38
In summary, Hellmich reiterated that the key goals of therapy in AAV are to control disease activity and prevent permanent organ damage and treatment-related AEs. For the induction of remission, a reduced steroid protocol (in combination with standard rituximab or cyclophosphamide) is equally effective but benefits from fewer infections. We now also have the opportunity to use targeted therapy with avacopan, said Hellmich, which shows similar efficacy for induction of remission compared to GCs, with the added advantages of reduced relapse risk and a lower rate of GC-related AEs. For maintenance of remission, rituximab is superior to azathioprine, with treatment beyond 18 months affording further reductions in relapse risk.