Meeting Summary
In a highly interactive symposium, a multidisciplinary faculty from across Europe assembled to discuss how best to meet the expectations of patients with rheumatoid arthritis (RA) in an increasingly complex therapeutic landscape. The introduction of biologic therapies and, subsequently, their biosimilars have been of great importance in improving treatment outcomes and have had a considerable impact on many healthcare economies. As more biosimilars are approved, the expert panel discussed how patients with RA can be treated more effectively during the early window of opportunity, which may lead to sustained remission, prevention of structural damage to bones and joints, and provision of more quality-adjusted life years to patients while simultaneously offering major savings for healthcare systems.
Introduction
Anti-TNF biologics have revolutionised treatment of RA. More recently, the emergence of high-quality biosimilars has provided a cost-effective means of prescribing biologic therapies to eligible patients. However, clinical challenges persist including practicalities of switching, from both a patient and physician’s perspective, in addition to dealing with healthcare economic systems to ensure that eligible patients have access to the most effective treatment in a timely manner. Patient expectations are at the very centre of decisions regarding disease management, and meeting these is a key feature of measuring treatment success. In this symposium, the basis
for drug selection in an increasingly busy landscape was discussed. The introduction of biosimilars, which offer patients with rheumatic diseases earlier and more sustained treatment, increasing the probability of long-term remission with less cost constraint was highlighted. Best practices for managing and meeting patient expectations in different healthcare economies were considered by an expert, multidisciplinary faculty from across Europe.
Treatment Choice in Rheumatoid Arthritis: The Therapeutic Landscape
The current therapeutic landscape in RA was outlined by Prof Taylor, who described the evolution of treatment. A generation ago, the only available disease-modifying antirheumatic drugs (DMARD), such as methotrexate, leflunomide, sulfasalazine, cyclosporine A, gold, hydroxychloroquine, and glucocorticoids, were of the conventional synthetic type (csDMARD). Biologic therapies were first approved in the late 1990s; however, largely due to cost constraints, many healthcare economies do not offer the early use of biologics, even when csDMARD do not achieve the therapeutic target of disease remission.
Prof Taylor noted the complexity of biologic therapies due to their size, the need for living organisms to produce them, and tightly controlled manufacturing process when compared with small-molecule drugs. He therefore emphasised that different batches of the same biologic exhibit great similarity but are not exact replications.
With patents for reference biologic therapies expiring, biosimilars have emerged on the market. Approval processes, including the demonstration of their biosimilarity, are rigorous, and there are abundant data supporting equivalence in terms of the efficacy and safety of biosimilar molecules compared with their reference products. Prof Taylor said that, in light of this, a challenge faced by prescribers is deciding which biosimilar to select for a patient, especially while choice increases as new products become available.
What are the Drivers of Drug Selection?
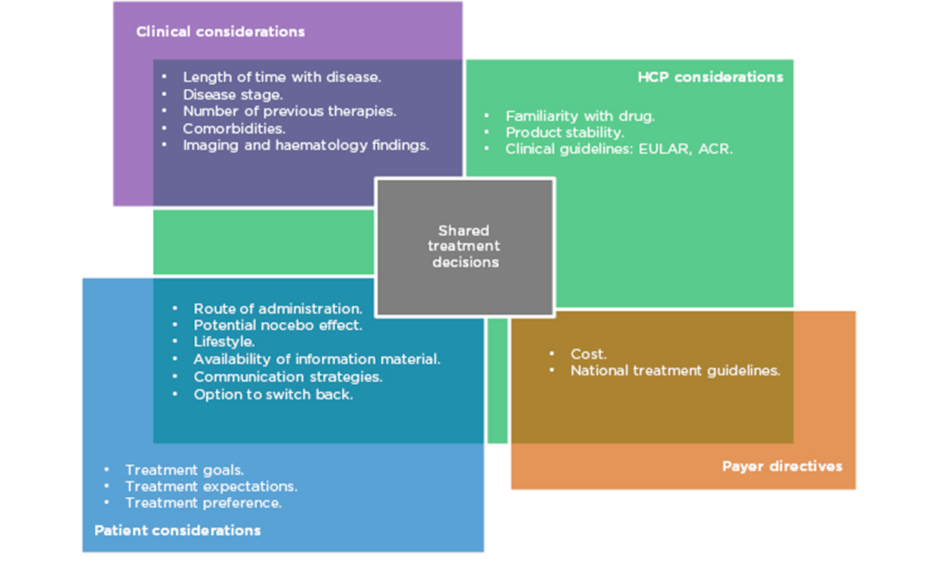
Prof Taylor outlined aspects of treatment that need to be considered when prescribing in RA (Figure 1). Biological factors include the length of time with disease, disease stage and activity score, number of previous therapies, and existing comorbidities. More established disease can be associated with comorbidities such as cardiovascular disease, lung and ocular involvement, inflammatory bowel disease, and depression. As such, treatment must be chosen carefully to avoid development or exacerbation of the aforementioned.
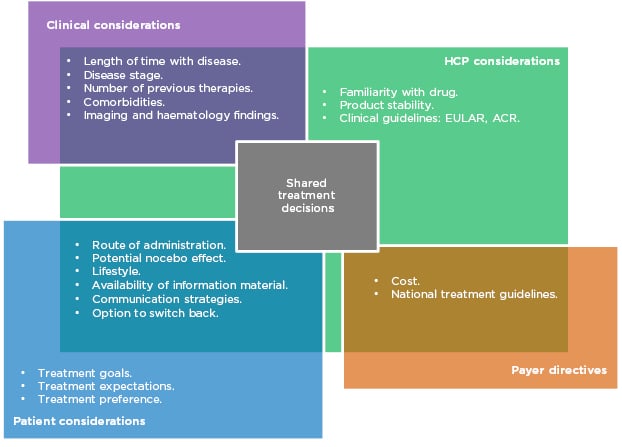
Figure 1: Many factors in multiple domains overlap to influence treatment decisions in RA.
ACR: American College of Rheumatology; EULAR: European League Against Rheumatism; HCP: healthcare professionals; RA: rheumatoid arthritis.
Patient choice is also an important consideration. Their preference of administration route and their individual lifestyle are factors that should be taken into account, particularly concerning whether or not they are comfortable using needles. Prof Matucci Cerinic expressed the need to listen carefully to the patient’s expectations for treatment and to make an informed, shared decision. However, it is also necessary to evaluate the safety, tolerability, and immunogenicity of each drug, along with efficacy and adherence rates.
While all of these are factors in the decision-making process, cost is also a major driver of treatment selection and often limits the selection of the most appropriate treatment in a timely manner. Profs Matucci Cerinic, Müller-Ladner, and Thomas confirmed that in their experience in Italy, Germany, and France, respectively, authorities are concerned about the expense of prescribing. Different countries’ healthcare economies have different guidelines, but all limit treatment options to the most cost-effective therapies to some extent.
An audience poll revealed that cost considerations were the most important non-medical factor influencing their treatment decisions (40%), although this was closely followed by familiarity with a drug (33%). Prof Müller-Ladner commented that drug familiarity entailed knowledge of its price and how the product works, and is important not only for physicians, but also for others involved in care including nurses and the patients themselves.
Choosing Between Biosimilars of the Same Reference Product to Meet Patient Expectations
Prof Müller-Ladner discussed the crowded anti-TNF biosimilar landscape, with five adalimumab, three infliximab, and two etanercept products currently approved by the European Medicines Agency (EMA), with many more in development. He reiterated the concept of demonstrating similarity in both non-clinical and clinical studies comparing products sourced from multiple countries before being approved for use. This increasingly busy field of nearly identical biosimilar products makes differentiating between them difficult.
Product attributes such as physical, chemical, and biological stability have implications for the selection of biologic medications. In a study involving 255 patients, only 7% of participants stored all biological DMARD (bDMARD) packages within the Summary of Product Characteristics (SmPC)-recommended temperature range.1 Different adalimumab biosimilars have different approvals for storage duration both within and outside the cool chain; a longer, approved stability outside the cool chain may be beneficial to patients who travel for extended periods of time, for example. Other differences between currently available adalimumab biosimilar products include needle gauge, injection volume, presence of citrate in the formulation, and latex components in the device. The delivery device can be a pre-filled syringe or an autoinjector pen, and different marketed products have unique characteristics. Prof Müller-Ladner noted that these small variations may make a significant difference: a slightly larger needle gauge may cause more pain to the patient and reduce their adherence, and a latex allergy will disqualify certain patients from certain products. He recommended that if a patient fares less well on a particular therapy, the physician returns to a consideration of all features of a particular product to find one that the patient prefers.
Prof Müller-Ladner suggested that flexibility is also important in selecting the administration device and that patients should be given an opportunity to return to the clinic if their expectations are not being met. He also remarked that physicians themselves may never have seen the devices that they are prescribing for patients and recommended that all healthcare professionals (HCP) familiarise themselves with the physical delivery devices. This, he noted “is one of the little details of prescribing behaviour that you can refine to benefit your patients.”
Prof Thomas spoke about the importance of the confidence that prescribing physicians have in biosimilars. Although it is an integral part of the doctor’s role, he identified a particular need for transferring confidence during a switch from a reference product to a biosimilar and in “giving a fair explanation” for this occurrence. This strategy may be helpful in setting patient expectations at a reasonable level prior to commencing therapy, thereby increasing the chance that they will be met.
The Nocebo Effect: When Patient Expectations Affect Outcomes
The fact that biosimilars are mostly prescribed for cost-saving reasons may lead a patient to believe that there is a reduction in quality that comes with this, meaning that they are potentially more vulnerable to being associated with a nocebo effect. When a patient has negative expectations of a therapy, psychogenic adverse events or lack of efficacy may be noted.2
Prof Taylor described a Finnish study in which patients were started on a biologic treatment shortly after they began to emerge onto the market for the first time.3 Functional and disease activity scores (DAS) were measured. The results were compared with those from another cohort who had started the same therapeutic 10 years later. DAS and functional scores were comparable, but a discrepancy was seen in the patients’ satisfaction with the treatment, with patients in the initial cohort reporting significantly higher satisfaction levels compared with the second treatment group. It is suggested that this was due to increasing patient expectation.
In an audience poll, 39% of respondents reported identifying a potential nocebo effect in <20%, and 23% reported seeing it in >20% of their patients. A total of 19% of respondents were not sure whether they had encountered it and the same proportion reported that they had not. Language and mannerisms used in communication are equally important. ‘Positive framing’, which involves reassuring the patient and sharing data reinforcing efficacy alongside adverse event information, can be employed to instil further patient confidence in the treatment and prevent negative expectations, therefore mitigating a potential nocebo effect.
Individual words, too, can have an impact on outcomes via the nocebo effect. For example, for some patients, ‘cheaper’ may connote inferior quality and, therefore, describing biosimilars in terms of ‘cost-effectiveness’ could help to reduce nocebo effects. Body language, too, can influence a patient’s reaction to a new medication.4
An audience member asked how it is possible to be certain that any problem is a result of the nocebo effect and not an issue with the drug itself. Prof Taylor suggested that, by virtue of being a biosimilar, a therapy will have demonstrated equivalence to the reference product in rigorous preclinical and clinical trials. He pointed to further evidence of expectation bias bringing about a nocebo effect in other studies involving switches between non-biologic drugs in different therapy areas.5-7 Nevertheless, Prof Taylor noted that even with cohort-level evidence, there remains a responsibility to treat every patient individually and to consider alternatives if a patient is not responding optimally having switched to a biosimilar.
Providing Switch Information: Setting Reasonable Patient Expectations
Echoing Prof Matucci Cerinic’s sentiment, Ms Slack agreed that, in an ideal world, all treatment decisions would be shared between the physician and the patient, with all therapy options available to choose from. However, similar to the situation in the other countries represented, health authorities in the UK have restricted biologic prescribing and issued directives to switch all patients on biologic drugs to biosimilars. Because of this, Ms Slack suggested that it was a situation “not so much about informed decisions, but about informed consent” to switch. She highlighted the importance of openness with the patient about the reasons for the switch, and making all the relevant information accessible to the patient.
How information is communicated to patients is crucial. Ms Slack referenced the position statement from the UK National Rheumatoid Arthritis Society (NRAS) regarding switching. Ideally, patients should have a face-to-face consultation with their HCP to discuss the switch, including the reasons, risks, and benefits. Prof Matucci Cerinic said that when his centre switched all patients from a reference product to its biosimilar, a successful strategy was to have a doctor reserved for the purpose of individually discussing switching with patients. He suggested that this proactivity was, in part, responsible for inspiring confidence and led to very high uptake rates, patient co-operation, and improved adherence rates.
Where individual consultations are not possible and the information must be communicated in writing, the reason for the switch should be made clear and a telephone number for queries should be provided. This was relevant to a question from an audience member who practised in Colombia and described her clinical practice, in which she may see 30 patients in a single morning. Prof Taylor also mentioned his centre’s ‘patient education sessions’, where patients are able to ask questions to nurse specialists and talk with other patients to share experiences, and recommended this as an effective way to bring patients and clinicians together for discussion in a time-efficient manner.
HCP who are open, accessible, and can speak frankly about the switching process and reasons for it transfer confidence to patients and help to build trust in their treatment. This could lead to greater switch acceptance and adherence rates. Healthcare institutions are encouraged to prepare ‘One Voice’ packages, which provide standardised lexicon and language usage guidance for all staff to ensure that a unified and coherent message is given to the patient. Ms Slack reported that, in her experience, patients had inherent trust in their HCP and would usually not query medical issues surrounding the switching process; however, they were more concerned about the practical aspects. Concurrently, she said that, from her own experience, she would be reluctant to assure patients that assenting to a switch would have immediately tangible
benefits, such as being able to fund additional clinical staff in the department. If the healthcare economic system in question did not proceed to reinvest savings directly then this could lead to patient expectations not being met. She emphasised the importance of reassuring patients that they would be able to switch back to the reference product if they felt that treatment with a biosimilar was leading to inferior outcomes. In a state-directed switching programme at her centre in the UK, she reported that only 3 out of 200 patients refused a switch, but that the assurance of the option to return to the initial drug was an important factor in obtaining consent.
The Therapeutic Window of Opportunity: Halting Disease Progression
Prof Matucci Cerinic presented a case study of a patient with early-stage RA who presented with high levels of circulating rheumatoid factor and anti-citrullinated protein antibodies. Sonographic imaging also indicated high disease activity. Current EULAR and American College of Rheumatology (ACR) recommendations suggest that csDMARD treatment should be commenced “as soon as the diagnosis of RA is made,” with the aim of bringing about clinical remission. He presented data indicating that early csDMARD treatment significantly reduced progression of radiographic joint damage since symptom onset.8 The faculty discussed current treatment algorithms. Prof Taylor noted that in patients with such poor prognoses, it would be ideal to introduce bDMARD into the combination therapy regimen as soon as possible. Prof Müller-Ladner suggested that the aim of treatment in this case should be “to stop the fire from spreading to the rest of the body,” and said that “in this kind of patient, one should be allowed to have a combination right away.” He added that it is always possible to remove bDMARD or csDMARD from the combination, but unfortunately, economic constraints in individual countries mean that this treatment is not available. This does vary between countries, however, and Prof Thomas noted that in France it is possible to introduce bDMARD into combinations early on, though not as first-line therapy.
In a poll, 75% of audience members agreed that the current treatment algorithms should be modified to allow use of biologics earlier in therapy pathways assuming cost is not an issue. An audience member asked whether “clinical guidelines should be driven only by clinical outcomes and not by cost… since that means that the right drug was being withheld based on economic considerations.” Prof Taylor suggested that, beyond an ethical issue, it was also a societal and political one. He described the process in the UK, where the National Institute for Health and Care Excellence (NICE) must make decisions, on behalf of the country’s entire population, on how to disseminate limited funds in the most beneficial way for all patients with all medical problems. He noted that making policymakers aware of ethical complications could initiate change, but that funds would have to be diverted from another source to secure this. Another audience question referred specifically to the position of NICE, and whether the body would change its position based on the more cost-effective nature of biosimilars. Prof Taylor confirmed that, currently, patients must have a 28-joint DAS of at least 5.1 before being considered for therapy with targeted agents, but that NICE were currently reviewing RA treatment guidelines and that this may change.
Therapeutic Drug Monitoring: Optimising Patient Management
During the meeting, the use of therapeutic drug monitoring (TDM) to inform decisions about therapeutic dose adjustment (dose tapering or dose intensification) was also discussed. Again, it was agreed that these considerations are usually driven by healthcare economics and not by dose limiting. Prof Taylor said that treat-to-target has revolutionised care, allowing for individualised treatment; however, most biologics do not have a dose-titratable range within licence. It may be possible to consider TDM as a means of pharmacological dose optimisation.
Prof Thomas outlined the reasoning and methodologies employed in TDM,9,10 and presented data from the RETRO study.11 This was a randomised controlled trial in which 101 patients with RA in stable remission continued DMARD treatment at the same level, tapered down to 50% of the original dose, or ceased the treatment after 6 months of the tapered dose. In the tapering or ceasing cohorts there was a significantly higher rate of relapse from the sustained remission disease state. However, at 1 year, approximately 60% of patients in the tapering cohort and 50% of those in the treatment cessation cohort remained in stable remission. Prof Thomas mentioned recent publications in which TDM was evaluated as a tool to inform successful tapering12 and to optimise treatment selection in patients who had lost response to adalimumab.9 If sufficiently high circulating drug levels are present following dose reduction then it is more likely that a patient will remain in sustained remission, suggesting that TDM may be helpful in facilitating cost-effective treatment strategies.
Prof Matucci Cerinic recalled clinical scenarios in which patients were keen to taper or stop therapies, often requesting that methotrexate treatment be tapered or stopped instead of biologic treatment. He said that patient wishes would need to be taken into consideration, and agreed with an earlier proposition made by Prof Thomas suggesting a strategy of ceasing steroid and tapering methotrexate treatment prior to stopping biologic therapy. He also indicated that ending steroid treatment is often most beneficial for the patient in terms of adverse effects, and may therefore help to meet patient expectations effectively.
Ms Slack presented a case study on dose tapering, focussing on the expectations of the patient. Patients themselves are more likely to see their disease in terms of their quality of life and daily activities, not by numerical, clinical scores. In this case, a 56-year-old male had a number of personal goals that he wanted to achieve through treatment, including being able to climb stairs and cycle. After 6 months of treatment on methotrexate and treble DMARD therapy, he still had high health assessment questionnaire scores and DAS and was therefore eligible for biologic treatment.
Following 4 months’ biologic treatment, his DAS was significantly reduced, he had returned to work part time, and had achieved all his personal goals. He expressed a desire to reduce the burden of his regimen, and the nursing team advised him on a strategy for this: reducing methotrexate and csDMARD dosages while maintaining the bDMARD regimen, correlating with the recommendations of Prof Thomas and Prof Matucci Cerinic earlier in the symposium. Ms Slack described discrepancies between recommendations and real-life practices. While guidelines suggest that tapering should only be undertaken in patients exhibiting sustained remission with imaging also displaying low disease activity, treatment decisions ultimately lie with the patient, and she noted that patients do often taper their own medication without prior HCP engagement. HCP should be wary, therefore, that patients may look to meet their own expectations without a discussion with their treatment team. Equally, HCP who openly and proactively discuss treatment preferences with patients can foster increased adherence rates and better outcomes.
Conclusion
The introduction of efficacious biologic treatments has revolutionised therapeutic pathways for the treatment of RA and, accompanying their more widespread use, there has been increased patient expectation for treatment success. More cost-effective biosimilars have the potential to reduce the financial burden on health economies and to allow effective biologic therapies to be introduced earlier in treatment pathways, but challenges may be involved relating to patient expectations. Looking forward, the introduction of biosimilars may spark discussion among policymakers and medical societies and serve as an opportunity to recommend earlier use of biologics to best exploit the vital therapeutic window of opportunity. The possible place of TDM as a tool to optimise treatment with biologics might aid in sustaining responses and making informed treatment selection decisions, while also reducing financial burden. The introduction of biosimilars offers optimisation of RA treatment, and the availability of more effective therapies increases the likelihood of patients finding a treatment that suits their lifestyle and meets their expectations.