Meeting Summary
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is an umbrella term for a group of rare, multisystem autoimmune disorders characterised by inflammation and damage to small blood vessels throughout the body.1,2 Management is centred on immunosuppressive/immunomodulatory approaches, with glucocorticoids (GCs) providing a key cornerstone of treatment.3 However, a clear gap exists between current clinical practice and treatment guidelines in the management of AAV, which can adversely impact both patient outcomes and quality of life (QoL). During this symposium, leading vasculitis experts considered the key challenges of managing patients with AAV in the real-world, focusing on the need to balance disease control against the known clinical risks associated with prolonged and high-dose GC exposure. A potential future role for complement-targeting agents within the AAV treatment paradigm was also discussed, based on new insights into complement-driven disease pathogenesis. These agents may help address an important unmet need which currently exists within AAV for effective therapies with an improved safety profile.
Ideal World Versus Real-World: Vasculitis Remission and Risk of Relapse in Europe
Neil Basu
Systemic vasculitis is the most heterogenous of all disorders, with the numerous different subtypes classified according to blood vessel size.4 Granulomatosis with polyangiitis (GPA) is defined pathologically by necrotising granulomatous inflammation, and is associated with antibodies to proteinase 3, while its sister condition, microscopic polyangiitis (MPA), is associated with antibodies to myeloperoxidase.4 Both diseases can affect any part of the body but have a predilection for the ear, nose, and throat system, lungs, kidneys, skin, and joints.
Outcomes for patients with MPA and GPA have been transformed over recent decades, although premature mortality is still evident compared with the general population.5,6 This transformation in vasculitis mortality can be largely attributed to cyclophosphamide, explained Basu: an important drug, but one that carries serious complications such as infections and cancers if used long term. Several multicentre randomised controlled trials have therefore evaluated ways of using cyclophosphamide more intelligently, including the pivotal CYCAZERAM study in which long-term cyclophosphamide therapy was replaced with azathioprine.7 In this European trial, 155 patients with AAV who had reached remission with cyclophosphamide were randomised to either continue cyclophosphamide or switch to azathioprine.7 After 18 months, no difference in relapse-free survival was seen between the two arms.7 Other studies have explored strategies to further minimise cyclophosphamide dose, including the CYCLOPS trial that compared oral with intravenous cyclophosphamide and found both approaches to be similarly effective in controlling AAV.8
As well as azathioprine, there are other maintenance agents in the toolbox to help maintain remission, continued Basu. The French WEGENT trial demonstrated similar efficacy of methotrexate to azathioprine in maintaining relapse-free survival in AAV, while the EUVAS IMPROVE trial showed azathioprine was superior to mycophenolate in relapse prevention.9,10 More recently, a number of trials have also confirmed a key role for rituximab as a maintenance agent. The MAINRITSAN study showed rituximab 500 mg 6 monthly was superior to daily azathioprine in maintaining remission, while the RITAZERAM trial, which focused on relapsing patients using 1 g rituximab every 4 months, confirmed this superiority.11,12 It is important to note that, in both trials, relapse rates began to accrue again after rituximab therapy was completed.
Results from key randomised controlled trials have been synthesised into evidence-based AAV treatment guidelines; however, Basu stressed that problems still occur in this ‘ideal world’ guidelines-directed management of patients.3 Relapse remains a common event, with combined follow-up from EUVAS trials showing a 50% relapse rate at 7 years.13 Premature mortality also poses an ongoing issue, not because of the disease itself, but events such as serious infection in the first year, and cardiovascular disease and cancer thereafter.6 In the ‘ideal world’ it is difficult enough trying to balance the fear of disease with the fear of drug toxicity, explained Basu, but in the ‘real-world’ clinicians face numerous additional challenges, including drug access, lack of multidisciplinary team care, diagnostic delays, and patient comorbidities.
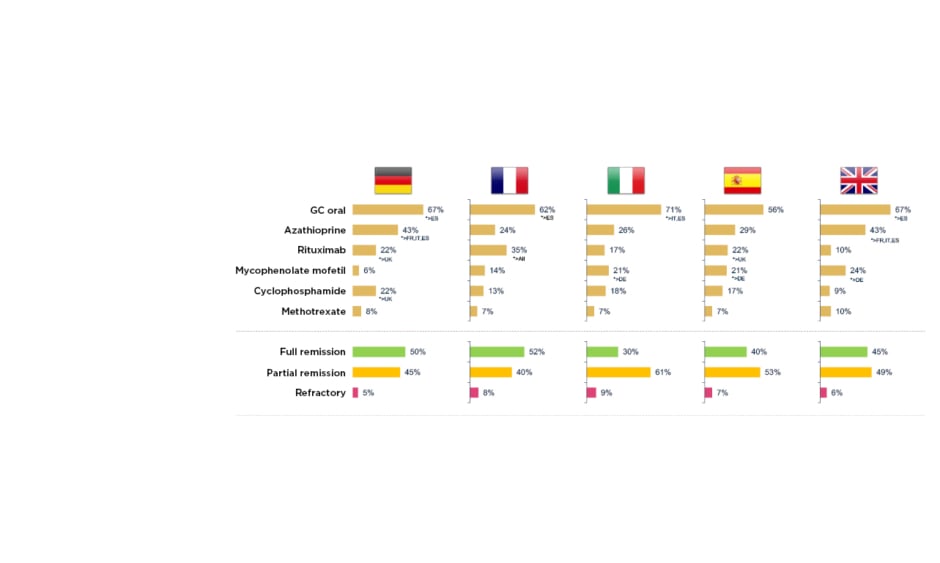
To examine this real-world AAV treatment landscape, a retrospective, observational study was recently carried out across five European countries.14 It involved 493 physicians from the European Union (EU) and 1,478 patients with AAV, followed from induction to 36 months maintenance, of whom 51% had MPA and 49% GPA. Mean patient age was 54.2 years, 56% were male and 44% exhibited severe progressive disease. The profile of drugs used for induction therapy was found to be similar across different countries, mostly cyclophosphamide and rituximab, although Basu described it as ‘surprising’ to see up 15% of patients receiving steroids alone for induction. At the start of maintenance, around 50% of patients were still not in full remission, in contrast to evidence from clinical trials which suggests that approximately 85% of patients achieve full remission by 3–6 months (Figure 1).14 He suggested that this disconnect could be explained by different definitions of maintenance used by physicians and inter-country differences in practice, for example, 22% of patients were receiving cyclophosphamide in Germany at the start of maintenance compared with only 9% in the UK.14 After 36 months of maintenance, this real-world study revealed that a significant number of patients had still not experienced full remission, and some remained on long-term cyclophosphamide (e.g., 12% in Germany). Use of high-dose oral steroids for prolonged time periods was also a common theme across the vasculitis disease course, with around 20–40% of European patients remaining on GC doses ≥7.5 mg for up to 36 months.14
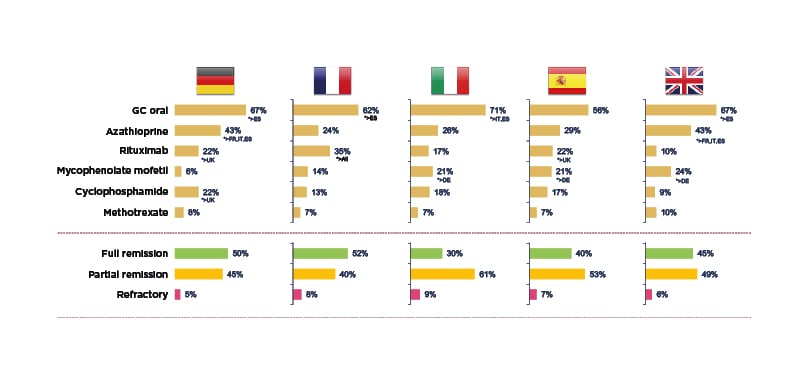
Figure 1: Real-world clinical practice study: treatment and outcomes at the start of maintenance in AAV.14
*Base: maintenance patients DE=300, FR=278, IT=300, ES=300, UK=300. DE: Germany; ES: Spain; FR: France; GC: glucocorticoid; IT: Italy.
In another insight into real-world AAV outcomes, Basu shared data from the Scottish AAV Linkage study in which 563 patients with classifiable AAV from different regions were identified and matched with general population controls.15
Compared with controls, patients with AAV exhibited an excess risk of numerous comorbidities including osteoporosis, cardiovascular disease, diabetes, and hypothyroidism. Infections rates were also higher in patients with AAV versus controls over an 8-year period, with the increased risk of infection most pronounced at diagnosis when patients were receiving induction treatment. The cumulative effect of comorbidities also proved greater than their sum, noted Basu, as evidenced by the degree of excess multimorbidity amongst patients with AAV in this study over time compared with the matched general population.15
Looking at the impact of vasculitis on real-world patient QoL, a recent UK-based study evaluated QoL, measured by the Short Form Health Survey with 36 items, in 470 patients with AAV versus 318 matched controls.16 Across most domains, patients with AAV had significantly inferior QoL compared with the general population and were 2.5 times and over 11 times more likely to experience poor mental and physical QoL, respectively.16 This impairment in QoL in patients with AAV appears comparable with that of other significant chronic diseases such as RA, lupus, and dialysis. Poor QoL in AAV can be driven by multiple, complex factors including clinical factors such as steroids and psychosocial factors like depression, anxiety, and fatigue.16
Heterogeneity of practice in the management of AAV also exists, as evidenced by a recent systematic review and meta-analysis of 10 studies that found significant variability in mortality data across different cohorts.17 These disparities may stem from differences in levels of specialisation, the degree of multidisciplinary team involvement, and availability of fast-track access.
Overall, Basu concluded that a disconnect exists between current clinical practice and treatment guidelines in the management of AAV, which may relate to both heterogeneous disease and practice. Despite significant process, patients with AAV therefore continue to experience premature mortality, excess multimorbidity, and poor QoL.
Drivers of Disease in AAV: Pathophysiology, the Role of Complement, Treatment, and What Comes Next?
Peter Lamprecht
Peter Lamprecht explored disease drivers in AAV, focusing on the role of complement activation and inhibition. The complement cascade can be activated by three different pathways, classical, lectin, and alternative, leading to generation of so-called anaphylatoxins, C3a and C5a, which have the ability to activate immune cells via interaction with key cell-surface receptors.18 Lamprecht highlighted the activation of neutrophils via binding to the C5a receptor as particularly critical to the pathogenesis of AAV.
AAV itself is a small-vessel vasculitis in which neutrophils adhere to the endothelial cell layer and degranulate, inducing vessel-wall necrosis.19 Looking more closely at the underlying pathogenesis, Lamprecht pinpointed neutrophil-expressed proteinase 3 (PR3) and myeloperoxidase (MPO) as the “prime autoantigens of disease.”20 Against a background of genetic predisposition and environmental factors, an immune response against these autoantigens is induced in patients with AAV.20 Plasma cells then generate ANCA, which interact with MPO and PR3 on the cell surface of cytokine-primed neutrophils.20 This induces neutrophil activation close to the endothelial cells, a highly pathologic event leading to endothelial cell damage and subsequent vasculitis.20
For a long time, the role of complement in the immune processes leading to AAV was unclear but experimental models have helped to shed light on its key pathogenic role. The first evidence for the role of complement in AAV was provided by a murine glomerulonephritis (GN) model in which MPO–ANCA generated in MPO knockouts was transferred to wild-type mice and was shown to induce crescentic GN.21 Similar experiments demonstrated induction of crescentic GN using C4 knockout mice.21 As C4 is part of both the classical and lectin complement pathways, these findings confirmed that neither pathway is needed for induction of GN with MPO–ANCA.21 In contrast, there was no induction of GN by MPO–ANCA when the experiments were repeated in both factor B and C5 knockout mice, providing proof that the alternative pathway and complement C5 are essential.21
An extension of this model was used to confirm the key role of the C5a receptor (C5aR) in a generation of GN, with C5aR knockouts showing no GN as compared with wild-type animals.22 Further experiments using mice carrying the human C5aR, treated with the inhibitory drug CCX168, showed induction of glomerular crescents by anti-MPO (39.3%), and anti-MPO and vehicle (30.5%), but very little crescentic GN with anti-MPO plus CCX168 (3.28); thus demonstrating that an oral inhibitor of the C5aR was able to ameliorate MPO–ANCA-induced GN.22
Other research has sought to decipher exactly how the alternative complement pathway is activated in AAV. In these experiments, normal TNF primed neutrophils were first incubated with IgG then the supernatant was reacted with normal serum. Activation of complement was measured by C3a generation, expressed as a percentage of the mean of the results for control IgG. Adding anti-MPO or anti-PR3 IgG elicited significantly higher C3a generation at 173% and 146% of control, respectively, whereas IgG from healthy controls had no impact.21 These findings suggest that stimulation of neutrophils by ANCA causes release of factors that activate complement via the alternative pathway.
In actual patients with AAV, different complement profiles are evident. For example, increased levels of factor Bb are seen in patients with MPO AAV in remission but not PR3 AAV. In contrast, high C5a levels are found in both active PR3 and MPO AAV, and C3a is elevated in the plasma in MPO and PR3 AAV during active disease and remission. C4d, a fragment of the classical pathway, appears elevated in PR3 AAV.23 Staining of human tissue for factor Bb, C3d, and C5b-9 has shown elevated levels in both the glomerula and small vessels as compared with non-AAV controls.24
Levels of factor H, a key inhibitor of alternative pathway activation, are decreased in active AAV compared with normal controls, but increase again when patients attain remission.25 Interestingly, factor H is also correlated with renal prognosis in AAV, with low factor H levels in the plasma associated with reduced renal survival.25 Research led by Lamprecht’s own group has shown that anti-C5aR antibodies, which occur naturally in the circulation, are decreased in patients with AAV.26 In a study of 110 patients with GPA and MPA, lower anti-C5aR levels were linked with higher disease activity, as denoted by the Birmingham Vasculitis Score (BVAS), and this correlated inversely with C5a levels in the plasma. Anti-C5aR antibody levels above and below the cut-off of 0.45 U/mL were also associated with major and minor relapses for both groups of patients with PR3–ANCA+ and MPO–ANCA+ AAV.26
Moving on to consider therapeutic targets in AAV, Lamprecht explained that classical treatment with GCs and cytotoxic agents inhibits lymphoproliferation and thereby modulates the adaptive immune response against ANCA targets, while rituximab offers more targeted B-cell depletion. New agents targeting the pathogenesis of AAV by specifically inhibiting the alternative complement pathway are also undergoing clinical development (Figure 2).
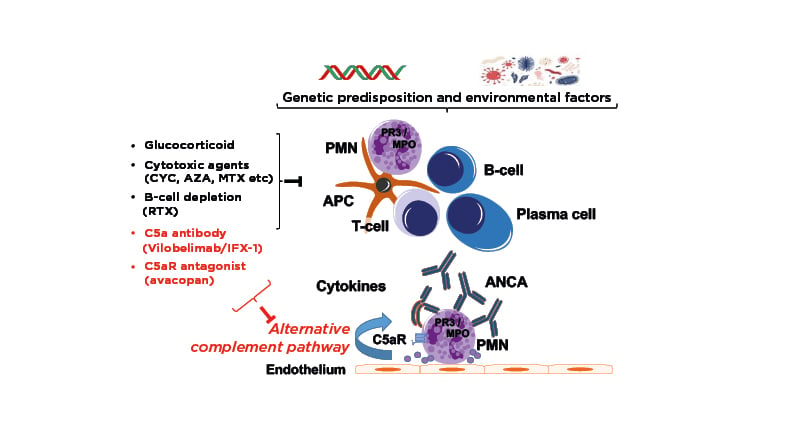
Figure 2: Pathogenesis and complement-targeting therapeutics in AAV.22
AAV: ANCA-associated vasculitis; ANCA: anti-neutrophil cytoplasmic antibody; APC: antigen-presenting cell; AZA: azathioprine; C5aR: C5a receptor; CYC: cyclophosphamide; MPO: myeloperoxidase; MTX: methotrexate; RTX: rituximab; PMN: polymononuclear neutrophil; PR3: proteinase 3.
Phase II trials of the C5a antibody vilobelimab (IFX-1) are currently recruiting27,28 and a published Phase III trial of the C5aR antagonist avacopan has shown it to be an effective GC-sparing agent in induction of remission in AAV.29,30 A range of other candidates targeting different factors in the complement cascade are also undergoing evaluation in Phase I–III trials in AAV and other glomerular diseases.29
Summing up, Lamprecht explained that ANCA-induced neutrophil activation results in endothelial damage and activation of the alternative pathway of the complement system, especially in MPO AAV, but also in PR3 AAV, which differ with respect to their complement profiles. Regulators of the complement pathway such as factor H and anti-C5aR antibodies are decreased in AAV, indicating strong dysregulation of the complement system. Moving forward, inhibitors targeting the complement system at different levels and pathways could offer important new therapeutic options for patients with AAV.
The Real-World: A Case Study on the Risks of Glucocorticoids
Aladdin Mohammad
Updated global data on the epidemiology of AAV indicate that both incidence and prevalence are continuing to climb.31 European prevalence currently ranks highest in Norway at 261 cases per 1 million population, followed by the USA at 218 and the rest of the world in the range 24–196.31 Prevalence represents an important measure of the global burden of disease and denotes the number of patients who require clinical care and good treatment options, explained Mohammad. Recent studies from across Europe also suggest that the age-specific incidence of AAV is shifting higher, with peak age at diagnosis now standing at over 75 years.31
GCs occupy a central pillar in current European League Against Rheumatism (EULAR)/European Renal Association–European Dialysis and Treatment Association (ERA-EDTA) treatment guidelines for AAV but a number of important questions remain unanswered, such as: what constitutes the optimal dose at induction and what is the best tapering schedule? The EULAR recommends tapering of prednisolone or equivalent to 7.5–10.0 mg by Week 12.3 However, in key clinical trials, the average dose of daily prednisolone was actually found to be 10 mg after 19 weeks and 7.5 mg after 21 weeks.3 This is in a trial setting where adherence to the tapering protocol would be expected as very good, stressed Mohammad; in real-life practice we are dealing with patients with repeated relapses and longer time periods of observation. He showcased results from his own hospital, illustrating the potential benefits of a reduced-dose GC schedule such as PEXIVAS which led to lower cumulative doses of oral GCs, with an average saving of around 2 g in the first 52 weeks of treatment.32
Other important outstanding questions relate to the threshold in dose safety for patients on long-term GC treatment and the need for methylprednisolone (MP) pulse therapy. The viewpoint from the EULAR task force is that GC doses ≤5 mg/day pose an “acceptable low level of harm” but at >10 mg/day the risk of harm becomes elevated.33 At intermediate doses, risk remains uncertain and patient-specific characteristics need careful consideration.33 On the role of MP pulse therapy, findings from a retrospective USA/European study of 114 patients with newly diagnosed severe AAV found no difference in survival in patients who received MP pulse therapy, but a significantly higher incidence of infections, severe infections, and new-onset diabetes compared with those who did not.34
Mohammad went on to outline some of the key evidence, demonstrating the impact of long-term GC exposure on patients with AAV. Data from 296 patients involved in four EUVAS trials revealed that mean length of GC use was 40.4 months, in stark contrast to guideline recommendations to taper/stop steroid by Month 6.35 A high level of organ damage was independently associated with increased cumulative GC use (p=0.016) and patients with longer duration of GC use were more likely to have a total Vasculitis Damage Index (VDI) score >5.35 Severe treatment-related damage was linked to both frequency and duration of GC use.35 Another important aspect to consider is the extent and pattern of organ damage in AAV. A population-based study of 86 patients followed for a median of 9 years showed that real-life treatment-related damage occurred frequently and was significantly more common in older patients (>65 years).36 A further retrospective study from three European countries focusing specifically on elderly patients ≥75 years at diagnosis found that cumulative MP dose was associated with treatment-related damage (odds ratio: 1.25) and cumulative oral prednisolone dose was predictive of death due to infection.37 Osteoporosis, cataracts, and diabetes all consistently ranked among the leading manifestations of treatment-related damage due to GCs across the various studies presented.35,37
Addressing the impact of GC exposure on the occurrence of comorbidities, Mohammad discussed data from two population-based, matched-control studies. Rate ratios of comorbidities were shown to be significantly higher for patients with AAV versus controls, most notably osteoporosis (rate ratio: 4.6–8.0), diabetes (rate ratio: 2.0–2.1), and hypertension (1.4–2.4), and occurred early during the disease course.38,39 GC therapy was also found to be one of the key factors associated with severe outcomes (odds ratio: 3.7) in a multicentre UKIVAS cohort study involving 65 patients with systemic vasculitis diagnosed with COVID-19.40
In summary, Mohammad noted how the epidemiology landscape of AAV is shifting towards higher prevalence and more patients are living with these diseases; many of them elderly. GCs constitute one of the cornerstones of treatment, but a number of important clinical questions remain unanswered, most notably around the balance of dose and risk. What is certain is that prolonged use of GCs is associated with higher rates of severe organ damage and comorbidities, worse outcome from infections, and increased mortality. GCs undoubtedly have beneficial effects in AAV treatment regimens, Mohammad concluded, but an important unmet need persists for alternative therapies with improved efficacy and safety.