Meeting Summary
Despite considerable advances in treatment, only one-third of patients with rheumatoid arthritis (RA) achieve clinical remission.1 RA is a multidimensional inflammatory disease; in addition to joint manifestations, most patients have systemic involvement or other comorbidities contributing to poor outcomes such as disability.2 Are there missing links in RA patient management that lead to suboptimal disease control, and prevent health providers’ good intentions from translating into favourable outcomes?
The aim of Looking beyond the joint in RA (LiBRA) 2019 was to identify the missing links in clinical practice and explore how best to address them for improved disease control and patient outcomes. The faculty examined the multistep pathogenesis of RA, with consideration of genetic and immunological drivers of the disease and the environmental and lifestyle factors that help shape disease phenotypes. Opportunities for timely intervention and patient-tailored management were explored, with discussion of how best to measure disease activity to guide therapeutic decisions. The disconnect between patients’ and physicians’ perceptions of disease can drive treatment nonadherence, and beliefs and biases of both patients and healthcare professionals (HCP) can negatively influence behaviours and outcomes. Practical approaches to overcoming barriers to adherence and physician best practice, including collaborative goal setting and shared decision-making, were explored from a behavioural science perspective.
TREATING THE RIGHT PATIENT, AT THE RIGHT TIME, WITH THE RIGHT DRUG: IS IT POSSIBLE?
Here the faculty explored the progression and pathobiology of RA to identify opportunities for more effective intervention. The session addressed how rheumatologists can best utilise the disease-modifying treatments now available, and how disease management could be tailored to individual patients.
Defining Clinical and Treatment-Response Phenotypes in Rheumatoid Arthritis by Molecular Pathology: Towards Precision Medicine
Professor Costantino Pitzalis
In managing patients with RA, one size does not fit all. Patients differ clinically in their disease evolution and outcomes, and in their treatment responses.3 Currently, rheumatologists cannot reliably predict the progression of early undifferentiated arthritis to early RA, evolution of early to established RA, or the therapeutic response at a given disease stage. Today’s trial-and-error approach to therapy selection is far from optimal and risks under or over-treatment. Improved understanding of the heterogeneity of RA pathobiology will inform development of clinically useful predictive biomarkers and bring precision medicine in RA a step nearer.
There are currently no clinically useful peripheral blood biomarkers to inform individually tailored treatment;4 could looking within the joint be more informative? Synovitis is the hallmark of RA and the first manifestation of disease localisation to the joint, sometimes following years of systemic immunological dysfunction.5 RA synovitis can lead to degradation of cartilage and bone, although the time-course of joint destruction is highly variable.3 Synovial biopsy research is providing insights into the diverse pathotypes (cellular and molecular signatures) of synovial tissue that might underlie the divergent clinical phenotypes of RA and could lead to a clinical tool for patient stratification.3
An integrated pathobiology-driven patient stratification research programme is in progress in Europe, involving patients with RA of various stages and treatment experience. Synovial biopsy tissue is analysed pre and post-treatment to identify the histological and molecular expression patterns associated with response and disease evolution. In an early RA treatment-naïve cohort, comprehensive RNA-sequence analysis of synovial tissue and peripheral blood, linked to in-depth phenotypic profiling, has identified synovial transcriptional subgroups associated with three distinct synovial histological pathotypes and diverse clinical and treatment response phenotypes.6,7 A workstream of the Maximising Therapeutic Utility for Rheumatoid Arthritis (MATURA) consortium is identifying peripheral blood biomarkers associated with synovial molecular signatures.8
The Window of Opportunity: What Are We Missing and When?
Professor Ernest Choy and Professor Janet Pope
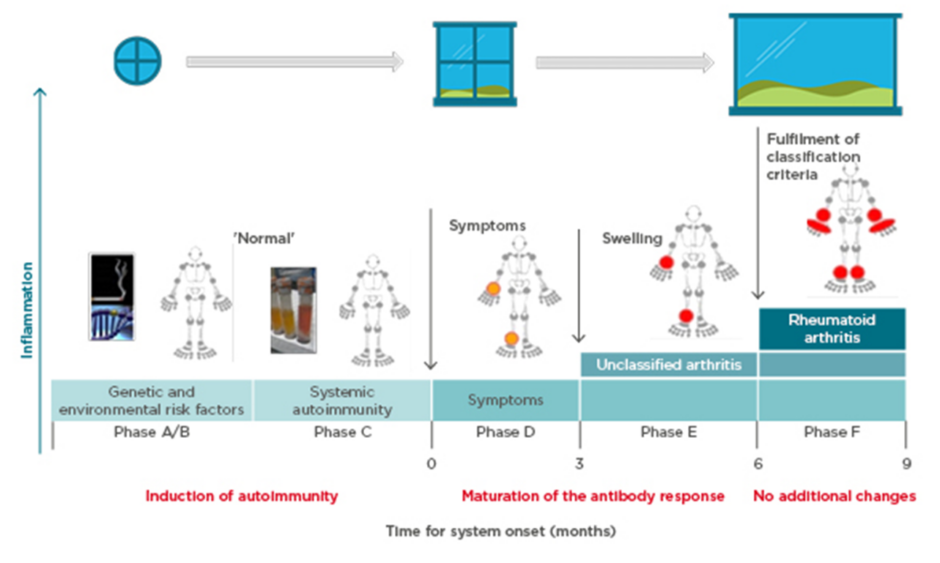
Prof Choy explored the pathogenesis of anticitrullinated protein antibody (ACPA)-positive inflammatory arthritis from onset through to chronic refractory RA and proposed that more than one ‘window of opportunity’ for effective intervention may exist at different timepoints (Figure 1).9 Autoimmunity in RA is triggered through presentation of citrullinated peptides to human leukocyte antigen Class II alleles, leading to T-cell activation. A combination of genetic (e.g., HLA–DRB1 alleles, PADI4) and environmental (e.g., smoking, infection) risk factors provide the background for this breakdown in tolerance,10-12 offering a hypothetical window for intervention at this initial preclinical stage. Affinity maturation drives epitope spreading and generation of proinflammatory ACPA, amplified by bacterial infections.13,14 Symptoms of early arthritis develop, providing access to the patient and a more relevant window of opportunity for timely diagnosis and treatment initiation, followed by a wider window once RA classification criteria are fulfilled. However, by the time the most effective treatments are used, the immune response may already have amplified to an irreversible degree.15
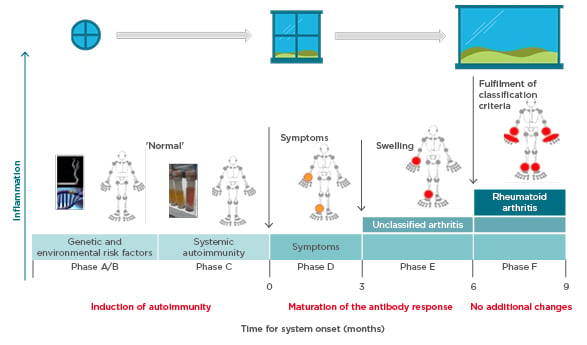
Figure 1: Windows of opportunity in rheumatoid arthritis pathogenesis, aligned to the six phases of preclinical and earliest clinically apparent rheumatoid arthritis.9
Prof Pope identified potential missing links in management from when the patient first presents with symptoms. Lifestyle factors should be targeted at this early stage; in pre-RA ACPA-positive patients, high BMI and smoking increase the risk of progression to RA if the shared epitope is present.16,17 Other predictive factors for development of RA include the number of swollen joints, acute phase reactant levels, ACPA and rheumatoid factor positivity, and imaging findings.18 The European League Against Rheumatism (EULAR) recommends early initiation of disease-modifying antirheumatic drugs (DMARD) in patients at risk of persistent arthritis, even if criteria for inflammatory rheumatologic disease are not met.18 Early referral to a specialist improves long-term disease outcomes,19 and starting DMARD within a 3–6 month window reduces the risk of joint damage and increases the likelihood of achieving sustained remission.20,21 However, delays are seen between presentation to primary care and rheumatology referral, and again to the first rheumatology consultation.22 Community case-finding strategies can help identify patients with early inflammatory arthritis, and early RA clinics provide a point of early access for rheumatology assessment.23
In established RA, comorbidities are a potentially modifiable missing link, given their association with difficult-to-treat disease and functional impairment.2,24 Missing links remain in our understanding of how to sequence biologics once conventional DMARD have failed.
Session summary
An era of stratified medicine in RA, with molecular pathology an integral part of management, is approaching. This will help realise the goal of selecting the right drug for the right patient, the first time, with the promise of safer, more efficacious, and more cost-effective therapy. Although precision medicine in RA is not yet a clinical reality, the Pathobiology of Early Arthritis Cohort (PEAC) study has already generated a unique interactive resource for research, linking detailed phenotypic information to synovial and blood gene expression data.7,25
Meanwhile, action is needed to tackle delays in referral and DMARD initiation for patients at risk of persistent arthritis, given evidence for a therapeutic window of opportunity of only 3–6 months at most from symptom onset.20,21 Early arthritis clinics, referral triage, and rapid access systems can facilitate early rheumatology assessment. Lifestyle modification is also important to prevent progression of early arthritis and RA. However, missing links in knowledge remain, particularly in the mechanistic pathways and intervention opportunities for non-ACPA-positive disease.
IS IT POSSIBLE TO TALK ABOUT OPTIMAL DISEASE CONTROL IN RHEUMATOID ARTHRITIS?
The session focussed on what is meant by optimal disease control in RA, and how disease activity should be measured to best guide treatment decisions. Uncontrolled inflammation leads to structural joint damage and disability, but other factors can also contribute to poor patient outcomes and reduced quality of life. Is clinical remission still the optimum treatment target, and can poor outcomes be predicted and prevented?
Minimally Important Differences to Aid in Making Treatment Decisions
Professor Daniel Aletaha
Clinical remission is the primary recommended treatment target in RA, with low disease activity an acceptable alternative in some patients.26 Remission is defined by its measurement scale. Disease activity score (DAS)-28 remission, based on C-reactive protein or erythrocyte sedimentation rate, suffers from a lack of specificity (even with adjusted cutpoints) compared with the Clinical Disease Activity Index (CDAI),27,28 and is dependent not only on efficacy but the type of intervention used, evident from discordance in remission versus American College of Rheumatology (ACR) response rates for cytokine and noncytokine-based therapies.29-31 The ACR–EULAR recommended Boolean criteria also have limitations, with most ‘near misses’ (patients who fulfil 3 of the 4 remission criteria) failing because of a high (>1) patient global assessment (PtGA) score, even in the absence of inflammation.32
How much change is clinically important for decision-making? The minimal clinically important difference, which is a patient perception of improvement, is dependent on baseline activity.33,34 In a Canadian Early Arthritis Cohort (CATCH), the optimal cutpoint for the minimal clinically important difference for CDAI improvement was a change of 12, 6, and 1 for patients with high, moderate, and low disease activity, respectively.34 Following a treatment initiation or change, 3 months is a key decision point to assess benefit; a pooled analysis of patient-level trial data showed that a major response at 3 months (e.g., ACR70, simplified disease activity index [SDAI] score of 85%, or EULAR good response) is needed to predict target achievement at 6 months.35
What Drives Chronicity in Rheumatoid Arthritis?
Professor Ernest Choy, Professor Janet Pope, and Professor Daniel Aletaha
Prof Choy explored predictors of physical disability, one of the most important RA outcomes. Inception cohort data has revealed four health assessment questionnaire (HAQ) trajectories, including a severe trajectory characterised by persistently high HAQ from baseline onwards.36 Older age, female gender, and worse DAS-28 were associated with worse HAQ progression across the trajectories.36 In a pooled analysis of patient-level clinical trial data, improvement in HAQ was less likely for patients with long disease duration or significant joint damage, an effect of chronicity not observed for disease activity measures.37 However, as might be expected, the contribution of joint damage to disability is much smaller in early RA than in established disease.38 The prevalent symptoms of pain, depression, and fatigue are also strongly correlated with physical disability (and with each other),39,40 as is the comorbidity burden.41 Inflammation contributes to each of these indicators of disability (Figure 2).38-42
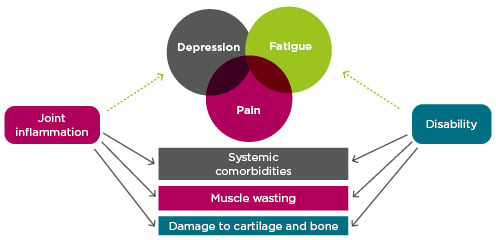
Figure 2: Indicators of disability in rheumatoid arthritis and the role of inflammation.38-42
Prof Pope considered the difference between residual disease activity (RDA) and residual inflammation in RA, and situations in which these misalign. RDA can be considered as the absence of clinical remission; however, disease activity assessment in RA is complex, with various measures and definitions of remission in clinical use. In a given patient, RDA will differ according to the measure and, for composite scores, its components, and the same score in different patients can mean different things depending on its components. Even identical subscores can be misleading; for example, swelling in two large joints is more important to patients and physicians than in two small joints. RDA does not necessarily entail residual inflammation because noninflammatory symptoms, such as residual pain, may result in a high PtGA score and some patients may never be able to meet composite remission criteria because of fibromyalgia or refractory pain that persists despite controlled inflammation.43,44 Conversely, patients who achieve clinical remission may still have residual inflammation.
Despite the limitations of disease activity scores and remission criteria, treating to target (i.e., adjusting treatment for those with RDA) remains important for patient outcomes. In the CareRA trial of patients with early RA, adherence to a treat-to-target (T2T) strategy resulted in an 88% remission rate, compared with 58% where T2T was not followed.45
Could imaging be a better way to detect residual joint inflammation? To date, imaging studies do not support routinely treating to a more stringent target of subclinical remission; however, imaging can help guide certain decisions, such as whether to taper treatment or whether to initiate DMARD in ACPA-negative patients.46-48
Refractory RA is an indicator of disease chronicity but has no single accepted definition. Prof Aletaha explored the meaning of refractory RA and whether it can be predicted earlier in the disease. Refractory RA could encompass various criteria: number of failed treatments, type/subtype of failed treatment, overall treatment duration, duration of each treatment, current use of corticosteroids, and level of disease activity (Figure 3). Of these, residual disease activity is an obvious requirement, and the number of failed treatments was another key criterion for most delegates. Studies are in progress to assess the heterogeneity of refractory RA and identify subtypes based on the presence, or absence, of inflammation or progression of joint damage (Aletaha, personal communication). In a cohort analysis of patients with refractory RA, defined as moderate/high disease activity, ≥3 treatment courses, ≥1 biologic, ≥18 months disease duration, and therapeutically amenable disease, longer time to initiate first treatment was a strong predictor of refractory disease.49 Women and patients with higher disease activity were also at increased risk of developing refractory RA.49
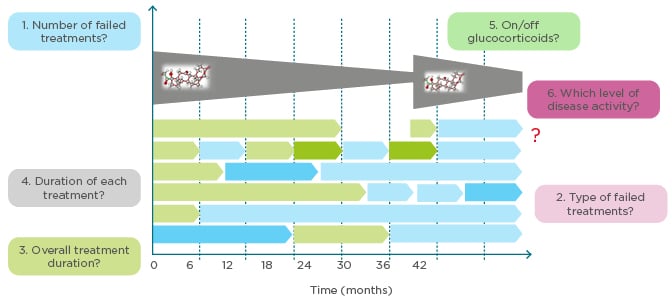
Figure 3: How to define refractory rheumatoid arthritis. What are the criteria for refractory rheumatoid arthritis?
Understanding and Managing Adherence in Rheumatoid Arthritis
Professor John Weinman
Nonadherence in general is a major public health problem, contributing to 200,000 early deaths each year in Europe.50 A systematic review of 52 studies of biologics in RA found adherence rates of 41–81%, and 1-year persistence of 32–91%.51 Nonadherent patients are half as likely to achieve remission, and take longer to do so, than adherent patients.52 The Capability, Opportunity, and Motivation (COM-B) model provides a useful framework for examining drivers of patient behaviour, specifically drivers of nonadherence.53 Patients’ beliefs (their perceptions of illness, treatment, and self-efficacy) help drive their motivation to take treatment;53 the least adherent patients have doubts about treatment necessity and concerns over potential adverse events.54,55 Beliefs can vary not only between patients, but in the same patient over the course of treatment.56 Research among HCP shows a lack of awareness of the adherence problem at an individual and system level, and many do not check for adherence in an effective, patient-friendly manner.50,57
Session Summary
Physicians should continue to strive for minimal inflammation, although patient dissatisfaction should prompt discussion of other treatment options. However, given that pain often drives near-misses of remission32 and noninflammatory pain is strongly linked to depression, assessing the impact of pain and depression before switching treatment may be advisable. Pain and depression, together with fatigue and comorbidities, are also strongly associated with physical disability, and in today’s era of effective disease-modifying therapies probably contribute more to disability than joint damage.
Despite the limitations of the available disease activity scores and remission criteria, treating to target improves patient outcomes. Evidence supports a target of clinical (rather than subclinical) remission, with the role of advanced imaging in routine care uncertain. Time to first treatment is currently the only modifiable risk factor for refractory disease, reinforcing the importance of early treatment intervention.
Nonadherence is an important missing link in RA management, with approximately half of patients not taking their treatment as directed.51 Physicians should facilitate informed adherence through shared decision-making, and by checking patients’ understanding of treatment and taking appropriate action where needed.57,58
COLLABORATIVE GOAL SETTING IN RHEUMATOID ARTHRITIS MANAGEMENT IN THE CONTEXT OF THE PATIENT–PHYSICIAN DISCONNECT
Patients and physicians can have very different perceptions of disease activity and management goals. This session explored the role of the patient perspective in evaluation of disease activity within a T2T approach, and implications for clinical decision-making. Discussion focussed on the PtGA, a component of the widely used composite disease activity measures and remission criteria, before broadening to consider the different types of patient-reported outcome (PRO) tools available and their value in daily practice. Delegates also learnt about two educational initiatives that are helping to address unmet needs and missing links in RA management.
Patient Perspectives in Definitions of Remission: A Critical Examination of the Patient Global Assessment
Mr Ricardo Ferreira, Professor Bruno Fautrel, Professor Daniel Aletaha, and Professor Janet Pope
Prof Aletaha reminded delegates that shared decision-making is a key component of RA T2T recommendations and management guidelines.59,60 However, patients and physicians bring very different perspectives, with patients highly concerned with pain, treatment toxicity, and the ‘fear of today’, and physicians more mindful of the disease and its consequences for long-term joint damage and disability. This manifests in discrepancies in patient and physician global assessments of disease activity; in patients starting treatment, key drivers of the PtGA/evaluator global assessment discrepancy are pain (driving a worse patient perception) and joint swelling (driving a worse physician perception).61 This disconnect has consequences for adherence; for example, if the patient feels well but faces a change in treatment or has pain but is kept on the same treatment they may not understand the purpose of treatment. Poor adherence, in turn, has a huge impact on many disease outcomes, including flare.62
Prof Fautrel argued for the importance of obtaining the patient perspective, for which the PtGA is the simplest measure, as part of routine disease activity assessment. Some studies have shown that retaining the PtGA within the Boolean definition of clinical remission does, to some extent, increase the predictive value of remission for radiographic progression (Ferreira, personal communication).63,64 The PtGA may also reflect meaningful, and outcome-predictive, fluctuations in disease activity between consultations.65,66 Even when the PtGA is driven by factors not related to active RA, such as mood deterioration or noninflammatory pain, this is useful information and provides a broader framework for physician intervention and access to other HCP as part of a more comprehensive and patient-centred approach.
However, the PtGA has limitations. The wording of the tool is not standardised, and Mr Ferreira described research showing that rates of remission, whether assessed using Boolean criteria or disease activity indices, vary by up to 6.3% according to which specific PtGA formulation is used, having potential implications for treatment decisions.67 The different formulations should therefore not be used interchangeably. Patients also have difficulty understanding the purpose and use of the PtGA, limiting its reliability. In a pilot intervention study, the rate of remission changed after patients received a structured explanation of the PtGA, although some patients provided invalid responses regardless of the intervention.68 A further limitation is that the long-term response of PtGA to treatment differs from that of other remission components such as swollen and tender joints and erythrocyte sedimentation rate.69
Inclusion of the PtGA within remission criteria can limit the achievement of remission for some patients, a problem not solved simply by increasing the PtGA cut-off for remission.70 Prof Pope showed data on rates of remission, and Boolean score ‘near remission’ (with PtGA >1), from the METEOR registry, indicating that three-times more patients would be in remission if the PtGA were excluded from the criteria.70 In a T2T approach, these near-misses could face treatment escalation despite lacking active inflammatory disease if the EULAR treatment guidelines are strictly followed.
Analysis by Mr Ferreira of the explanatory variables of the PtGA in patients with remission (defined by Boolean criteria including PtGA ≤1) and near-remission (Boolean criteria met except for PtGA >1) showed that the PtGA in near remission was driven by fatigue, pain, function, and anxiety, rather than by indicators of active disease.71
What do Patient-Reported Outcomes Tell the Clinician?
Professor Ennio Favalli
Prof Favalli summarised available tools for measuring the PRO in core outcome sets for rheumatic diseases and their value in clinical practice.72 Pain is important to patients and commonly reported despite a good response to DMARD.73 While a visual analogue scale provides a quantitative measure,74 a qualitative tool (short-form McGill or painDETECT) can help differentiate between types of pain and guide treatment.75,76 Function is usually measured using HAQ,77 which correlates with radiographic progression and predicts long-term outcomes;78,79 however, HAQ is time-consuming and performs poorly in patients with low disability.80 The HAQ-II tool addresses these limitations but is not yet widely used.81 Global assessment is usually performed using the unidimensional PtGA or Global Health measures,82 but multidimensional measures, for example the ACR-recommended patient activity scale (PAS), PAS-II or routine assessment of patient index data-3 (RAPID-3), or the EULAR-recommended RA impact of disease score (RAID), may be more informative,83,84 and could be useful for remote monitoring of stable patients. Morning stiffness is an important symptom with no standardised tool; a good approach is to combine duration and severity measures.85 Fatigue is difficult to resolve,85 and again can have multiple causes besides inflammation.86 The functional assessment of chronic illness therapy-fatigue (FACIT-F) scale provides the most informative measure.87 Measures of workability include arthritis-specific and nonspecific tools.88 Assessment of sleep disturbance and depression is complex as currently there is no clearly superior tool.89,90 The broader concept of health-related quality of life can be measured with the generic 36-item short-form healthy survey (SF-36) or EuroQol-5D,91,92 or with short forms of the ACR-recommended arthritis impact measurement scales (AIMS/AIMS2).93 PRO can support reporting of response to therapeutic interventions in clinical trials, as recently demonstrated for studies of IL-6 and JAK inhibitors,94,95 as well as in real-world practice.
How Can the eRA Programme and the Quality of Care Project Address Unmet Needs and Missing Links in Rheumatoid Arthritis Management?
Professor Bruno Fautrel, Professor Zoltán Szekanecz, and Ms Alison Kent
The evolving the management of RA (eRA) programme is an educational initiative designed to address unmet needs in RA management, led by an expert steering committee and supported by Sanofi Genzyme (Paris, France). Fourteen educational tools have been created for local use and adaptation across Europe, to support rheumatologists across five identified areas of need: 1) treatment initiation within the window of opportunity; 2) a T2T approach in daily practice; 3) encouraging shared decision-making and engaging the multidisciplinary team; 4) supporting identification and monitoring of comorbidity; and 5) sharing quality educational information for patients. Tools include educational slides, best practice guidance, clinical checklists, self-reflection questionnaires, case scenarios, and an interactive infographic for patients. An accessible web platform to support dissemination is now live.96 Localised websites for each country will follow, containing all country adaptations and translations.
The Quality of Care project, supported by Sanofi Genzyme (Paris, France), in collaboration with KPMG (Amstelveen, the Netherlands), is designed to support the adoption of good practice in the management of RA and associated comorbidities across Europe. The project was performed by KPMG, who reviewed the literature to identify challenges across the patient journey and visited 12 European medical centres to observe and document high-quality care. Examples of best practice were identified, prioritised by an expert steering committee, and published in a best practice report. In total, 18 good practice interventions have been identified across 3 patient profiles: suspected RA, recently diagnosed RA, and established disease/structural damage. Each intervention in the report is supplemented with case studies to support implementation.
Session Summary
The patient–physician disconnect can lead to poor adherence, with huge implications for patient outcomes, and understanding the disconnect is the first step towards addressing it. The disconnect can also affect whether remission criteria are met, which in turn affects treatment decisions. That the patient perspective is important to inform overall disease management and improve patient outcomes is not in doubt. However, in the view of some of the faculty, the PtGA should be excluded from definitions of clinical remission to avoid risk of overtreatment, and the patient perspective evaluated separately using appropriate tools. Remission should be redefined by identifying the most appropriate biological measure for the absence of active synovitis. In a T2T strategy, two targets may be required: a measure of inflammatory disease activity, and a target tailored to the patient’s personal goals.97,98 Pain is a highly important symptom and must be managed, with the understanding that suppression of inflammation may not eliminate pain. Various tools are available to fully evaluate pain and other important PRO in routine practice.
BEHAVIOURAL SCIENCE WORKSHOP: HOW CAN WE ACHIEVE BETTER DISEASE CONTROL AND OUTCOMES IN RHEUMATOID ARTHRITIS?
This session explored the changes in behaviour among both physicians and patients that are needed to improve patient management and outcomes in clinical practice. How can HCP change their behaviour and practice to better identify and manage suboptimal disease control, and to better support patients to communicate their disease burden and be active partners in decision-making?
Introductory presentations provided a background to the current theory of behaviour change and the impact of physicians’ attitudes, beliefs, and practices on patient management. In two interactive workshops, delegates then explored the barriers that prevent HCP from identifying and managing suboptimal disease control and gained skills in practical motivational interviewing techniques that can facilitate the patient–physician conversation on disease burden.
Principles of Behaviour Change
Professor John Weinman
Simply telling people what to do is rarely effective in eliciting behaviour change. Today’s field of behavioural science is based on the premise that any behaviour has multiple influences (determinants), and that to change the behaviour we need to understand the determinants, and possible barriers, and find the appropriate interventions to address them.99 The COM-B framework can help inform behaviour change interventions,53,100 which must be specific and individualised. In total, 93 behaviour change techniques have been defined,101 many of which can be applied to the healthcare setting. However, we should remember that behaviour change is a process, and can take time; therefore, anticipating barriers and monitoring progress is important to success.99
Leveraging Behaviour Change
Doctor Grace Wright
How do physicians’ attitudes, beliefs, and practices impact patient management and outcomes, and how can physicians adapt their own behaviours and ultimately those of their patients? Dr Wright focussed first on the challenge of effective listening. In an often-quoted study, patients were interrupted by their physician after an average of just 23 seconds, losing opportunities to gain information important for treatment decisions and to build empathy and trust.102 Briefly reviewing patient charts before consultations, and asking open-ended questions, are other important and evidence-based elements of effective consultation management.
Effective decision management is another important challenge. The disconnect between physicians’ intentions and decision-making outcomes stems from clinical (therapeutic) inertia, defined as the failure to adjust treatment when clinically indicated despite knowledge of the guideline and its applicability to the patient.103 In a study of 538 RA patients with moderate-to-high disease activity, median times to DMARD adjustment and achieving low disease activity were 5 and 10 months, respectively.104 Therapeutic inertia also occurs in conditions such as hypertension in which the treatment target is relatively simpler,105 suggesting that the complexity of human decision-making to initiate change, rather than of the disease in question, underlies the inertia. Contributing to this is humans’ use of heuristics, mental shortcuts based on emotion rather than logic, as the basis of decision-making. Although heuristics can be helpful, they have several associated biases that may influence behaviour in ways that inhibit change.106,107 Therapeutic inertia can be addressed through self-awareness and education on the psychology of medical decision-making, audit and feedback systems on performance quality, and decision-support reminders linked to patient records.
ADDRESSING HEALTHCARE PROFESSIONAL BARRIERS TO IDENTIFYING AND MANAGING SUBOPTIMAL DISEASE CONTROL
In this workshop, delegates discussed barriers that prevent them from identifying and managing suboptimal disease control in their patients, and potential solutions. Hypothetical clinical cases were used to identify key aspects of best practice, encompassing prompt initiation of treatment, regular follow-up with therapeutic adjustment/escalation as needed, comorbidity assessment and management, disease education for patients, advice and support for lifestyle changes, psychological support, effective communication, and shared decision-making. Various barriers to best practice working, relating to patients, physicians, or systemic factors, were identified, and then approaches and tools to overcome the barriers were then considered (Figure 4).
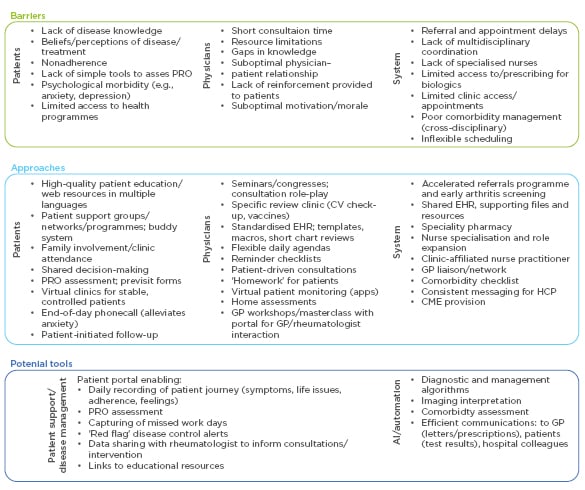
Figure 4: Barriers to best practice in rheumatoid arthritis management and potential solutions identified during a behavioural science workshop.
AI: artificial intelligence; CME: continuing medical education; CV: cardiovascular; EHR: electronic health record; GP: general practitioner; PRO: patient-reported outcome.
Tackling the Patient–Physician Disconnect: Facilitating Conversations on Disease Burden
Professor John Weinman and Ms Alison Kent
How can physicians best support patients to communicate their disease burden and goals, and share in decision-making? The second workshop began with an exploration of the barriers that, in the delegates’ experience, can prevent this dialogue. The identified barriers encompassed aspects of patient understanding, background and mindset, as well as the physician mindset and healthcare system barriers, such as lack of time for consultations.
Ms Kent introduced the concept of patient-centred coaching in healthcare and the role of the TGROW model in facilitating collaborative goal setting and behaviour change.108 The TGROW approach is of particular benefit in consultations with patients who have a real desire to change a health behaviour, perhaps to quit smoking, lose weight, or adhere to treatment, but who are struggling to make the change. The model provides a framework for patient-led conversations around five core elements: topic (initial agenda setting), goal (desired outcome), reality (understanding where the patient is currently in relation to their goals), options (explore what is possible for the patient moving forwards), and will (what will the patient do differently before the next consultation?).
Effective communication skills, encompassing aspects such as active listening, open questions, affirmations, reflections, and summarising are fundamental to the TGROW approach.109 As with any skill, communication can be improved. For the remainder of the session, motivational interviewing trainers equipped delegates with practical communication skills techniques to facilitate the patient–physician conversation.
Towards Holistic Rheumatoid Arthritis Management: Physicians’ Attitudes, Beliefs, and Practices
Doctor Grace Wright
The discussions have highlighted multiple missing links in RA management that contribute to suboptimal disease control including biological factors (disease phenotype and predictive biomarkers), systemic factors (referral and assessment delays), patient behaviour (lifestyle and adherence), disease activity assessment (choice of tools), HCP behaviour (clinical inertia), and the patient–physician disconnect. Fortunately, improved understanding of disease pathogenesis is providing opportunities to intervene at each stage of the disease course through earlier diagnosis, support for lifestyle change, outcome prediction, and timely and patient-tailored intervention to reach a treatment target.
Behaviour change in both patients and providers is needed. With approximately half of patients not taking treatment as prescribed, nonadherence is a huge concern and should be considered from the first consultation. Addressing adherence entails tackling the patient–physician disconnect by shifting to a culture of collaboration with genuinely shared decision-making and an agenda set by both parties but led by the patient. Behaviours are complex and change is difficult, with multiple barriers to overcome. By understanding the principles of behaviour change, and knowing how to optimise time with and effectively communicate with patients, physicians can facilitate change and demonstrate a concern for the patient’s perspective. Physicians must also understand the impact of their own attitudes, beliefs, and practices on patient care, including the heuristics and associated biases that govern decision-making and contribute to clinical inertia. By doing so, physicians can help to address missing links in RA management and achieve better disease control and outcomes in their patients.
RA is a multidimensional inflammatory disease requiring consideration of the totality of the patient’s morbidities, complications, and concerns. Holistic care is possible but requires effort to overcome therapeutic inertia. HCP must strive not only to treat the right patient, at the right time, with the right medicine, but to encourage patient self-management and self-advocacy through effective listening and empathy.