Chairperson: Georg Schett1
Speakers: Georg Schett,1 Ronald van Vollenhoven,2 Kunihiro Yamaoka,3 Maya Buch4
1. Universitätsklinikum Erlangen, Erlangen, Germany
2. Amsterdam University Medical Center, Amsterdam, the Netherlands
3. Kitasato University School of Medicine, Tokyo, Japan
4. University of Manchester, Manchester, UK
Disclosure: Prof Schett will receive an honorarium for this lecture from Galapagos NV and Gilead Sciences, Inc. Prof van Vollenhoven has received consulting and/or lecture fees from Bristol-Myers Squibb, GlaxoSmithKline, Lilly, and UCB; institutional grants from Pfizer and Roche; consultancy fees and institutional and/or personal honoraria from AbbVie, AstraZeneca, Biogen, Biotest, Celgene, Galapagos NV, Gilead Sciences, Inc., Janssen, Pfizer, Servier, and UCB; speaker, institutional, and/or personal honoraria from AbbVie, Galapagos NV, Janssen, Pfizer, and UCB; and will receive an honorarium for this lecture from Galapagos NV and Gilead Sciences, Inc. Prof Yamaoka has received consulting and/or lecture fees from AbbVie, Actelion Pharmaceuticals Japan Ltd., Asahi Kasei Pharma, Astellas Pharma, Boehringer Ingelheim Japan, Bristol-Myers Squibb, Chugai Pharmaceutical, Eli Lilly Japan, Daiichi-Sankyo, Eisai-Gilead G.K, GlaxoSmithKline, Janssen Pharma, Japan Tobacco, Mitsubishi Tanabe Pharma, Nippon Shinyaku Co Ltd, Ono Pharmaceutical, Otsuka Pharmaceutical, Pfizer, Takeda Industrial Pharma, and Teijin Pharma; and will receive an honorarium for this lecture from Galapagos NV and Gilead Sciences, Inc. Prof Buch has received consulting and/or lecture fees from AbbVie, Bristol-Myers Squibb, Eli Lilly, EMD Serono, Pfizer, Roche, Sandoz, Sanofi, and UCB; research grants paid to her employer from Pfizer, Bristol-Myers Squibb, Roche, and UCB; and will receive an honorarium for this lecture from Galapagos NV and Gilead Sciences, Inc.
Acknowledgements: Medical writing was provided by Bronwyn Boyes, London, UK.
Support: The symposium and publication of this article were funded by Galapagos NV
and Gilead Sciences, Inc. The views and opinions expressed are those of the presenters and not necessarily of Galapagos NV and Gilead Sciences, Inc.
GL-RA-FIL-202006-00002.
Citation: EMJ Rheumatol. 2020;7[1]:32-42.
Meeting Summary:
Prof Schett opened the session by explaining the overall theme and objectives of the symposium. Charles Darwin famously visited the Galápagos islands in 1835. His observations and collections of species of birds, also known as Darwin’s finches, showed the small physiology variations in the birds. Each bird species had a different food habit and lifestyle that led to the evolution and adaptation of different beak shapes and sizes.

These facts contributed to the development of Darwin’s theory of evolution by natural selection presented in his book “The Origin of Species by Means of Natural Selection.” These findings played a pivotal role in the formation of his scientific theories on evolution and natural selection. Similarly, the management of rheumatoid arthritis (RA) has followed in Darwin’s footsteps by evolving and becoming more complex, compelling innovative and collaborative solutions. The theory of evolution is not confined to animals and humans, but also provides a fundamental process in understanding diseases and there are several evolutionary chapters in RA. Research has advanced our ever-evolving and rapidly increasing understanding of RA pathology and molecular targeting which is flanked by a substantial and sustained development of new therapies leading doctors and patients to now have an expanding range of treatment options. This along with the progress in multidisciplinary treatment approaches; patients wanting to be actively involved in treatment decision making; the revolution of patient-centred digital communication using innovative, supportive technology; and the support of patient groups has led to the improved management of symptoms and better quality of life for patients with RA.
“In the long history of humankind (and animal kind, too) those who learned to collaborate and improvise most effectively have prevailed.” (Charles Darwin 1809–1882)1
How Rheumatoid Arthritis Therapy Has Evolved: From Humble Beginnings to Effective, Targeted Treatments
Professor Ronald van Vollenhoven
Prof van Vollenhoven discussed the evolution of therapy over the last 20–30 years, as well as the current and emerging paradigms of care for patients with RA. Firstly, RA clinicians have learned that the disease can be modified and not just treated symptomatically. This discovery has profoundly changed the disease. In addition, the development of highly precise tools has enabled reliable clinical assessment to ascertain the degree of inflammation, disease activity, radiological damage, and the impact on patient’s lives.
The understanding of the pathophysiological, inflammatory, and destructive processes at the molecular and cellular levels involved in RA has led to the development of multiple therapeutic options, including conventional, biological, and small-molecule JAK inhibitors.2-20
Over the past 70 years, treatment for RA has changed profoundly, evolving from a strategy of providing only symptomatic relief, to the realisation of regimens that impact disease activity and slowing or halting structural joint damage. Drug therapy for RA has evolved with improving efficacy and the impact on disease activity and radiographic progression, from gold salts in the 1930s to biologic response modifiers (biologic disease-modifying antirheumatic drugs [bDMARD]) (e.g., TNFα inhibitors, IL-1 inhibitors, B-cell inhibitors, T-cell costimulation inhibitors, and IL-6 receptor inhibitors) and finally to targeted synthetic DMARD approved in the last decade.7-20 When reviewing clinical trials with these different agents, it is important to realise that there have been both great successes and some failures (anti-CD4 inhibitors and spleen tyrosine kinase [SYK] inhibitors).21
The exponential development and availability of these improved therapeutic options, with different efficacy and safety profiles, were results of research and improved understanding of the RA pathology and disease.2-5,7,21 This, in turn, has changed the treatment paradigm facilitating consideration of patient choices, opinions, fears, and expectations, thereby compelling a more patient-centred treatment approach and22,23 enabling personalised treatment for patients with RA.
In addition to new drugs to treat RA, novel and reliable measurements have been developed to assess the outcomes of therapeutic intervention and have been incorporated into treat-to-target (T2T) approaches for managing RA. The tools for measuring disease activity have allowed us to reconsider the goals of treating our patients. Patients with active RA desire decreased pain and improved mobility and function. This translates into a need to control the inflammation with an overall goal of achieving a state of remission and sustained remission in those who can potentially achieve this. Low disease activity and sustained low disease activity is an alternative goal in those unable to achieve remission, particularly in long-standing disease.6,23,24
Two decades ago, Kirwan25 demonstrated that the initial correlation between inflammation and disability (e.g., pain and stiffness) is high and fluctuates with time (potentially attributable to the natural course of the disease or therapeutic interventions). As the disease progresses, radiographic damage develops and increases in correlation with disability and joint destruction, which becomes more relevant to the degree of disability experienced later in the disease process. Therefore, current treatment strategies target reducing inflammation and preventing, or limiting, radiographic damage to achieve optimal functional status with the least amount of disability for patients with RA. This involves early intervention with a proposed ‘window of opportunity’ varying from 3–6 months to the first 2 years.26,27
One of the most recent therapeutic developments for RA has been the development of JAK inhibitors. Studies comparing JAK inhibitors with anti-TNF agents have shown to be statistically significant superior or noninferior.28-30 For both patients and clinicians, it is exciting to have a class of agents available with this level of efficacy.
The significant evolution in understanding the underlying pathophysiological mechanism and development of new treatment modalities in RA has ultimately led to the need for early diagnosis, initiation of intensive therapy, and ‘tight control’ monitoring driven by regular measurements of disease activity. To achieve successful monitoring of the RA patient, there are two aspects requiring consideration. The first is to scientifically and objectively assess the degree of disease activity using instruments such as the Disease Activity Score 28 (DAS28), Simplified Disease Activity Index (SDAI), and Clinical Disease Activity Index (CDAI). These can be used along with the American College of Rheumatology–European League Against Rheumatism (ACR–EULAR) remission definition to monitor the patient’s remission status.31 Secondly, the patient’s health, quality of life, and functional status may be ascertained using one of the patient-reported outcomes such as the Health Assessment Questionnaire (HAQ), Routine Assessment of Patient Index Data 3 (RAPID3), Rheumatoid Arthritis Disease Activity Index (RADAI), Rapid Assessment of Disease Activity in Rheumatology (RADAR), or the Short Form 36 Health Survey (SF-36).32-34 It is important to balance the clinician’s goals of treatment with those of the patient by integrating current treatment strategies with a patient-centred approach. This involves seeing the patient as a unique individual and approaching the patient from a biopsychological perspective. These need to be viewed in the context of the environment of the patient (friends, family, and social support structure), their emotional wellbeing, and relationships.35,36 It is this approach to patient care that we must strive for if we are to meet the challenge posed by William Osler (1849–1919) over a century ago that: “The good physician treats the disease; the great physician treats the patient who has the disease.”37
“The good physician treats the disease; the great physician treats the patient who has the disease” – William Osler, 1849–1919
Indeed, many patients still do not reach the therapeutic targets, and many experience loss of response over time, despite innovative therapeutic strategies and assessment tools.6,38,39 In fact, real-world data from the Norwegian DMARD (NOR-DMARD) registry with 2,778 patients (Figure 1)38 found that less than 50% of patients achieved a strict definition of remission (DAS28-4; erythrocyte sedimentation rate <2.6) after 6 months of bDMARD monotherapy or combination therapy. In addition, almost 50% of patients stopped therapy after 24 or 60 months. Lack of efficacy was the most common reason for stopping treatment across all treatment groups. This was followed by adverse events.38 These data are further supported by other studies demonstrating that up to 50% of patients starting a new DMARD must stop it within 12–18 months,6 and in those who do achieve initial symptom control, only a few (11%) maintain sustained clinical remission by 5 years.40 One of the future goals of the rheumatology community is to achieve bDMARD-free remission, i.e., to start the patient on an advanced therapy to achieve disease control and then stop therapy as a result of sustained remission.
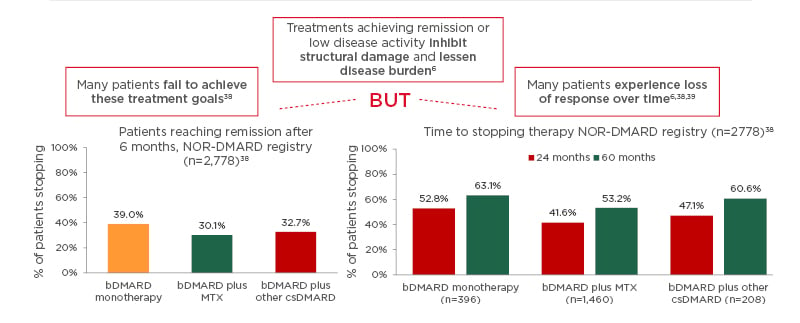
Figure 1: Many patients with rheumatoid arthritis fail to achieve treatment goals or experience loss of response over time.
Real-world data from the Norwegian DMARD (NOR-DMARD) registry analysing 2,778 treatment courses, including 396 biologic disease-modifying antirheumatic drug (bDMARD) monotherapies, 1,460 bDMARD plus methotrexate, and 208 bDMARD plus other conventional synthetic DMARD. There was no significant difference in efficacy between the bDMARD groups and the most common reasons for stopping bDMARD therapy were lack of efficacy, followed by adverse events.
bDMARD: biologic disease-modifying antirheumatic drug; csDMARD: conventional synthetic DMARD; MTX: methotrexate.
Adapted from Olsen et al.38
This is not easy to accomplish and it remains an enigmatic goal, as shown by Huizinga et al.41 who found that most patients (84%) who discontinued an advanced therapy had a subsequent flare of disease activity.
In the evolution of patient care in RA, there remain limitations requiring improvement. Even though many RA patients may not achieve the set therapeutic goals, they do not switch to alternative treatments because of concerns over toxicity of other treatments and accepting the status quo.38 Important symptoms, such as pain, physical function, and fatigue are not adequately assessed and addressed.42 Monotherapy as a general rule is less effective than combination therapy, with higher rates of stopping therapy with bDMARD monotherapy than with bDMARD combination therapy.38 Even with current T2T strategies a significant percentage of patients continue to have moderate to high disease activity.6,43 There is still a need for future therapies to enhance already established efficacy of current therapeutics and in patients who remain unresponsive to current treatments.6,31,43
In the future, we must adapt and learn to explore all the options and possibilities, including information technology and bioinformatics. Evolving technologies could enable extensive recording of real-time disease characteristics and molecular processes in individual patients to generate personal big data. Rheumatologists will require new strategies for the management of their patients to develop data-driven individualised concepts resulting in better diagnosis and treatment. These datasets could include devices to store data; genome typing to identify disease-associated genes; noninvasive imaging to assess inflammation; gene expression analysis to discriminate between states of viral, bacterial, or other inflammation; and proteomics and autoantibody analysis.44 These evolving, sophisticated, and rapid techniques provide us with optimism and excitement about positive future developments.
In summary, even though the field of rheumatology has evolved extensively over the years, there is still more we can do for our patients. We want to achieve remission for all patients, which means that we may have to treat them earlier. The management of patients with RA is a fluid and evolving concept that has developed over time. In the near future, and also in the longer term, we can anticipate exciting developments in our ability to help patients living with RA. This will in part be based on our evolving understanding of RA pathology and the integration of new identification and validation techniques, resulting in novel therapeutics. As a result of this more in-depth understanding and range of therapies, RA management strategies can become more patient-centred and individualised.
Whilst RA management is continually evolving, many challenges such as low patient adherence,
lack of effective treatment switching, and high disease activity despite individualised T2T strategies still exist.
Evolution of Molecular Targeting
Professor Kunihiro Yamaoka
Modern advances in medical treatment have greatly benefited patients living with RA. The development of even more effective targeted therapies could be compelled by further discovery of the disease’s molecular pathology.7,45-47
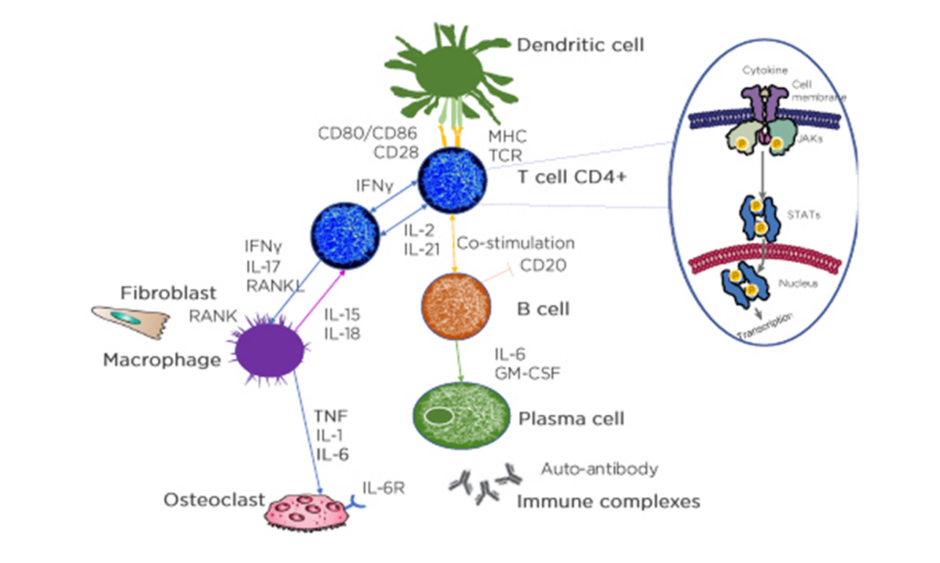
Looking into the histology of RA, some details are known about what is happening in the synovial fluid. RA is a complex disease that involves interactions between a variety of immune modulators and signalling pathways. The immune response consists of a series of communications between many cell types. Interactions between antigen-presenting cells (APC) and T cells may initiate and amplify T-cell-dependent immune responses. Immune modulators, such as cytokines, and cells of the immune system, including neutrophils, macrophages, T cells, B cells, plasma cells, and autoantibodies, all contribute to the pathophysiology of the disease, and ultimately, are responsible for the joint damage in RA.
The synovial tissue in patients with RA is enriched with mature APC and many T lymphocytes. Dendritic APC present antigens to T cells for activation, and activated T cells then activate B cells, which then differentiate into plasma cells or memory B cells. Cytokine production by APC and T cells includes receptor activation of NF-κB ligand (RANKL), IFNγ, TNFα, IL-2, IL-6, and IL-17. T cells can express NF-κB ligand which can differentiate precursor cells into bone-resorbing osteoclasts, which can lead to bone loss and disruption in the joints.3,48
In each cell affected by cytokines, the triggered cytokine signalling cascade runs in the cytoplasm and one of these is JAK. JAK is activated directly after the cytokine binds to its receptor and JAK activates signal transducer and activator of transcription (STAT), which, upon dimerisation, move into the nucleus and regulate the transcription of multiple genes (Figure 2).47
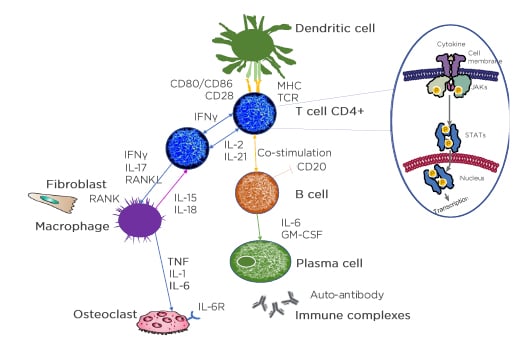
Figure 2: The understanding of pathological pathways has led to an array of treatment options.
Identification of molecular targets requires clear understanding of complex cytokine pathways.
APC: antigen presenting cells; CD: cluster of differentiation; GM-CSF: granulocyte-macrophage colony-stimulating factor; IL-6R: IL-6 receptor; MHC: major histocompatibility complex; RANKL: receptor activator of nuclear factor‑κB ligand; TCR: T-cell receptor; P: phosphorylation.
Adapted from Smolen et al.3 and Virtanen et al.47
With this immune response cascade, the JAK–STAT pathway is heavily involved in RA. This has led to the advent of the JAK inhibitors, which are quite different to the bDMARD drugs because these molecules are able to enter the cytoplasm to inhibit the activation of JAK.47 Individual cytokines interact with specific intracellular pathways. Other intracellular signalling pathways involved in RA include the MAPK, SYK, NF-κB, and P13K.48 The MAPK pathway has been extensively studied; however, a p38MAPK inhibitor has proven unsuccessful as a RA treatment option when compared to methotrexate.49 Theories for this include dose limitations as a result of toxicity, altered biodistribution of newer molecules preventing central nervous system penetration, incorrect isoform targeting, blocking downstream of the signalling pathway will not block upstream kinases, and kinases in the MAPK pathway (e.g., p38α) may have a regulatory role in the induction of anti-inflammatory cytokines.49,50 Similarly, SYK inhibitors have also had limited success as an RA treatment. Although blocking SYK with fostamatinib and MK-8457 did not demonstrate statistically significant ACR 20% improvement criteria scores versus placebo, there was a signal of improvement on osteitis, synovitis, and erosion, highlighting the need for upstream blockade of cytokine pathways.50-52
The JAK–STAT pathway has a key role in transmitting signals to the nucleus and inducing production of more cytokines and other factors.53 Excessive cytokine signalling via the JAK–STAT pathway leads to inflammation, autoimmunity, bone erosion, and cartilage damage, which are intrinsic to RA pathology.54-60 There are four members of the JAK family: JAK1, JAK2, JAK3, and tyrosine-protein kinase 2 (TYK2). Different individual cytokines signal through different pairs of JAK family members, and by activating diverse STAT pairs, they can selectively mediate a wide array of downstream signalling. These molecules sit docked on the intracellular tails of the receptor molecules embedded in the membranes of the cell, and they will pair up with either one of their own kind (homodimers) or with other members (heterodimers). JAK1, JAK2, and TYK2 are involved in signals by several cytokine targets in inflammatory conditions, including IL-6, IL-12, IL-23, granulocyte macrophage colony-stimulating factor, and IFN. Specific JAK and STAT pairs mediate the message propagated by different cytokine signals. Specific pairing of JAK determines the signal transmitted to the nucleus, and the output produced, namely, JAK3 in conjunction with JAK1 is an important component of signal transduction for cytokine receptors that utilise the common gamma chain such as IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21; JAK2 plus JAK1 plus TYK2: IL-6, IL-11, IL-13, IL-27, IL-31, IL-35; JAK2 plus JAK2: granulocyte-macrophage colony-stimulating factor, erythropoietin, thyroid peroxidase; JAK1 plus TYK2: IFNα, IFNβ, IL-10, IL-20, IL-22, IL-28; and JAK2 plus JAK1: IFNγ.53
Proteins including JAK and STAT require phosphate groups for activation. A common source of this phosphate is ATP, which transfers chemical energy within cells. JAK are phosphotransferases that catalyse the transfer of phosphate from ATP to various substrates. The transfer of a phosphate group from ATP to a JAK activates the JAK. Activated JAK pairs facilitate the phosphorylation of STAT. JAK inhibitors competitively inhibit ATP binding because of their ATP-like structure by reversibly binding to ATP-binding sites. Without phosphorylation, JAK proteins remain inactive and are unable to phosphorylate their relevant STAT proteins. STAT proteins are therefore unable to dimerise and translocate to the nucleus and the expression of physiological modulators are inhibited.47,61 Because JAK are key regulators of several cytokines that have been implicated in RA development and progression, they have been identified as potential targets for inflammatory diseases.50,53
Even though we have an array of available therapeutic options, there is still room for improvement in patient satisfaction rates in the treatment of RA. Several patient surveys have shown 32–77% satisfaction rates with current treatments and care.62-65 This highlights a need for further treatment options. Furthering our understanding of RA pathology can assist in improving treatment options and management. There remains a need to support researchers in identifying new targets in preclinical research, provide explanations to physicians for drug efficacy and safety outcomes seen in clinical practice, and provide patients with knowledge of RA disease to enable patient inclusion in treatment decisions. A longitudinal monitoring analysis of drug response at multiomics levels in the peripheral blood of patients with RA revealed that drug treatments alter the molecular profile closer to that of healthy controls at the transcriptome, serum proteome, and immunophenotype level.66 This study highlighted that is not simple to identify which patients would benefit most from specific treatments.
We need to expand our knowledge of RA pathology to further guide therapy choice and management by outlining which patient groups would benefit from therapies against each specific molecular target, thereby, enabling more personalised therapeutic strategies.
In summary, the identification of molecular targets requires a clear understanding of complex cytokine pathways. RA pathology is an elaborate and complex network of signalling and molecular pathways. Despite differences in the mechanism of action, current DMARD have similar response rates and there is an unmet need for improving treatment options for patients with RA. Recent advances in technology and management strategies have allowed for further understanding of RA disease. A better understanding of RA pathophysiology can lead to the discovery of new or improved therapies, e.g., JAK inhibitors, though further study is required to understand treatment safety and efficacy and identify which individual patients may benefit from which drug. This information is key to the evolution of a patient-centric approach in RA management to ensure that we can address the quality of life of the patient.
Evolving Trends in Treatment Decision Making
Professor Maya Buch
Outcomes in patients with RA have dramatically improved over the past two decades as a result of combined efforts of better disease activity assessment and diagnostic tools along with a better armamentarium of therapeutic options.2,3 This has allowed us to focus on a T2T strategy with a patient-centric approach.6 Achieving patient-centred care across the spectrum of therapy choices has also evolved over time. Historically, the most common consultation with our patients was a paternalistic decision-making model where the patient passively agrees with healthcare professional (HCP) recommendations. More recently, the informed decision-making model of enabling patient empowerment and autonomy, with the HCP providing information and the patient making informed decisions, and the shared decision-making model, where the patient and HCP share equal involvement with both parties having an active dialogue to express preferences underpinned by clinical expertise to reach a consensus on the agreed management route, have been developed.22 This notion has been advocated by various professional and organisational guidelines and recommendations, including the EULAR 2019 RA management recommendations.6
“Patients require access to multiple drugs with different modes of action to address the heterogeneity of RA; they may require multiple successive therapies throughout life.”
“Treatment of patients with RA should aim at the best care and must be based on a shared decision between the patient and the rheumatologist.”
“Patient education may increase adherence to medication… patient education forms the implicit and inseparable basis for shared decision-making.”
Personalised care requires both the selection of a tailored therapy integrated with the involvement of the patient in the decision-making process to ensure the best possible outcomes. Identifying real-life factors that drive treatment choice is essential to optimal patient care. The physician considerations include overall drug efficacy; targeting remission; rapid drug onset and initial response, convincing efficacy evidence-based, clinical trial data; and comorbidities and drug safety intersection.67 The patient considerations include the long-term drug use associated with ‘reliance’ and ‘dependence’, the occurrence of side effects, perception of alternative treatment options, and the psychosocial aspect of the emotional impact/psychological burden of removing/starting medication with the stigma of requiring long-term disease modification.68 Patients also have preferences for the mode of administration of drugs and these preferences will affect treatment decisions. The clinical factors remain centrally crucial in defining which drugs may be important, and the route of administration is important when tailoring to the individual patient. Oral agents are perceived as better, providing autonomy and independence and rapid onset of action; however, some patients are reassured by intravenous/parenteral preparations which provide the comfort/safety of the hospital environment and reassurance from HCP. Whereas subcutaneous injections could provide patients with the confidence of a drug.69,70 These psychological aspects and perspectives of a patient are important to convey and listen to when we engage within our consultation.
A survey found that a large proportion understands the benefit of goal setting in clinical practice showing alignment to the physician-driven T2T strategy. The survey also highlighted that physicians may not articulate the goals of the T2T strategy when consulting with patients and almost three-quarters of the patients suggested that the HCP had not discussed an approach that achieves goals.36 Therefore, it is very important to verbally articulate our thoughts to the patient.
From a physician’s perspective, there are several composite indices to assess the disease activity of RA. The DAS28 being one of the most well established, but also the SDAI, CDAI, and more recently the Boolean remission criteria. The overall cut-off values of these assessments are used as an indicator of treatment efficacy in a patient; however, it is important to understand what components drive these different composite indices. The DAS28 score is a complex formula of the tender joint count 28, swollen joint count 28, erythrocyte sedimentation rate or C-reactive protein value, and the Patient Global Assessment (PGA).31 The composite score transforms and weighs the component variables, resulting in a stronger influence of tender rather than swollen joints and a very high contribution of acute-phase reactant levels to the score, even within their normal ranges. Consequently, swollen joints can still be present during remission and drugs that interfere directly with acute phase reactant synthesis show exaggerated DAS28 rates. Conversely, patients may not achieve remission but have an absence of swollen joint counts.
It is important to realise the discrepancy between the total disease activity score and the components of what the patient is telling us.3
Data from the Vienna group (n=646 RA patients) reviewing the perceptions of RA disease activity, as quantified by the PGA and by the Evaluator’s Global Assessment (EGA), demonstrated the most significant determinants for the cross-sectional and longitudinal discrepancy between the PGA and the EGA are pain (75.6%) and swollen joint count (60.9%), respectively. Highlighting the importance of recognising how pain that is not related to the inflammation also inputs into the disease activity assessment, which can be uncovered with improved engagements with our patients.71 As the patient’s clinical profile changes, the patient’s expectations adapt, and the physician appraisal evolves. Therefore, the physician’s perception of risk–benefit profiling and appropriate treatment choices evolve over time.68
With the advent of targeted therapies, initially with biologics and more recently with the oral synthetically targeted ones, there has been a tremendous emphasis and utility of registry data to inform the safety aspects of these drugs. These have been of enormous value and there may be some equivalency with certain kinds of toxicity (there are differences in the safety profiles of treatment options).72 The safety profiles of drugs become more pertinent in the context of the comorbidities in RA which are associated with poorer outcomes in patients. Most patients with RA are affected by a number of associated comorbidities.
Comorbidities in RA are associated with increased morbidity and mortality, impaired quality of life and treatment response, and increased complexity of management and its costs.73,74 Because these comorbidities can change over time, the scenarios are constantly evolving. To successfully manage RA, comorbidities should be carefully considered and treated in addition to prescribing medications. Comorbid conditions may impact treatment regimens of RA, or the prescribed drugs may worsen the comorbidity. Physicians may also be forced to prescribe RA medications that exacerbate the comorbid conditions.75
What becomes evident to us is that integrated management of comorbidities in RA is needed to determine the best treatment option for each patient managed through a rheumatologist-led multidisciplinary approach.6,76 It is also increasingly recognised that the concordance between the patient and HCP can improve outcome through adherence. Adherence to medication in patients with RA is low, varying between 30 and 80%. Risk factors for the lack of adherence include comorbidities, complex regimens, poor patient–HCP relationship, perceived treatment benefit, and lack of patient knowledge. Patient/HCP conversations to improve adherence and outcomes should cover the diagnosis and prognosis of illness, the need for proposed therapy, risks and benefits associated with treatment, the patient’s personal beliefs, concerns about prescribed medication, and concerns for the course of therapy.77
The level of desire for involvement in treatment decision is unique to each patient and physicians should not assume that all patients desire an equal partnership in treatment involvement. Results from a study interviewing patients living with RA for more than a decade (n=20) showed that the majority of patients (75%) followed a shared decision model; however, the level of involvement varied within this group ranging from equal involvement from both sides to a more paternalistic decision model.22 Clinical expertise has to inform and underpin the patient and their education. It is important to know our patients and recognise which is their preferred approach to formulate the best treatment decision with them.
Whilst the holy grail of RA treatment may be biomarker- and pathogenesis-driven, when it comes to clinical implementation in our practice, the final treatment decision requires an integration of a multitude of factors (Figure 3).6 These factors include the pathogenesis-driven treatment (precision medicine), tailoring treatment paradigm/T2T (choosing appropriate target/knowing the target), patient perspective, and the comorbidities and drug safety intersection.6 It is only when we bring these factors together in an active dialogue with our patients that we achieve an optimal outcome.
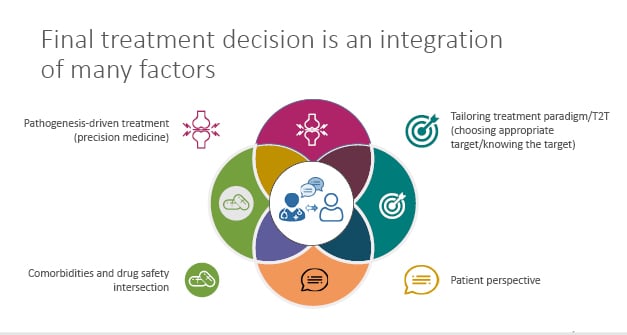
Figure 3: Rheumatoid arthritis treatment decisions requires integration of many factors.
T2T: treat-2-target.
Adapted from Smolen et al.6
In summary, the final treatment decision is an integration of many factors aiming to deliver optimal treatment outcomes. The contemporary management of patients with RA thus focusses on an integrated patient-inclusive approach. Improving communication barriers for patient information and education can promote the patient-centred integrated management approach of RA.
CONCLUSION
Over the last two decades, significant progress has been made in understanding the underlying pathophysiological mechanisms and treatment modalities in RA. These aspects have ultimately led to the unassailable need for early diagnosis, initiation of intensive T2T therapy, and tight control monitoring driven by regular measurements of disease activity. A combination of these aspects with a shared decision-making model, with an active dialogue to express preferences underpinned by clinical expertise to reach a consensus on the agreed management route, can result in significantly improved outcomes in RA patients.
CLICK BELOW TO VIEW THE FOLLOWING
Please visit https://hosted.bmj.com/in-darwins-footsteps
and https://www.congress.eular.org/ for more information about the symposium.