Meeting Summary
The symposium ‘Fine-tuning the treatment of PsA: Focus on the IL-23 pathway’ took place during the 2019 European League Against Rheumatism (EULAR) Annual Congress in Madrid, Spain. The presentations covered the rationale for targeting IL-23 in psoriatic arthritis (PsA), details of the IL-23 pathway relevant to psoriatic disease, practical implications and consequences of targeting IL-23, and experiences of targeting IL-23 in psoriasis from the dermatologists’ perspective.
Dr Stefan Siebert set the scene by outlining the pathophysiology of psoriatic diseases, particularly PsA, describing disease heterogeneity, explaining the role of inflammation, and highlighting the rationale for targeting the IL-12/23 pathway. He summarised key findings on the IL-12/23 inhibitor ustekinumab in PsA from clinical trials and real-world data available to date.
Delving deeper into the IL-23 pathway, Prof Georg Schett explained the function of IL-23 and its role in inflammatory disease and autoimmunity. After briefly describing the history of the relatively recent discovery of this cytokine, Prof Schett discussed preclinical and clinical studies underlying today’s understanding of IL-23 and why it is an appropriate target in PsA.
Multiple biologic or small-molecule treatments for PsA have been investigated in clinical trials. Prof Peter Taylor discussed the practical implications of targeting IL-23 and provided more details about the specific effects of targeting not only IL-23 (with risankizumab, tildrakizumab, or guselkumab) but also IL-12/23 (with ustekinumab) and IL-17 (with ixekizumab, secukinumab, or brodalumab).
In the final presentation, Prof Lluís Puig described clinical experience of targeting IL-23 in psoriasis and provided an overview of findings from several clinical trials, including: VOYAGE 1 and 2 (guselkumab versus the TNF inhibitor [TNFi] adalimumab); NAVIGATE (guselkumab versus ustekinumab); and the head-to-head ECLIPSE study (guselkumab versus secukinumab).
The symposium concluded with a lively panel discussion in which the speakers addressed a variety of questions and comments from the audience.
Introduction
The aim of this symposium was to familiarise participants with the IL-23 pathway and the rationale for targeting this pathway in PsA. Recent clinical and mechanistic data on targeting the IL-23 pathway in PsA were presented, and the real-world impact of these data on clinical practice was discussed, along with the effect of targeting the IL-12/23 pathway. Further aims were to increase participants’ understanding of how data on targeting the IL-23 pathway in psoriasis relate to the treatment of PsA, and to highlight unmet needs in the management of PsA in the clinic.
The Promise and Delivery of Targeting the IL-12/23 Pathway
Doctor Stefan Siebert
Psoriatic disease is extremely heterogeneous, with inflammation affecting the skin, joints, axial skeleton, and entheses.1 Genome-wide association studies have shown that psoriasis, and PsA in particular, are not only clinically but also genetically heterogeneous, yet the IL-12/23 and IL-17 pathways are implicated in the pathogenesis of both diseases.2-4 For example, increased IL-23 expression triggers a T cell response (stimulating IL-22 and IL-17 expression) in the entheses that leads to osteoproliferation, inflammation, and both bone loss and ankylosis in mouse models.3,4
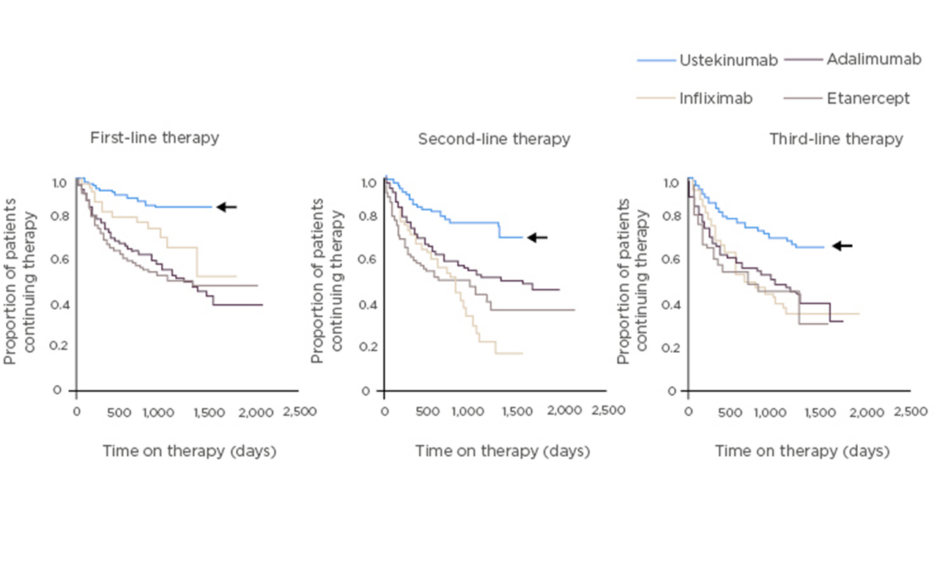
Efficacy of IL-12/23 inhibition by ustekinumab, which blocks the p40 subunit common to these two cytokines, has been demonstrated in both PsA (in the pivotal Phase III PSUMMIT studies) and psoriasis.5-9 Integrated safety data from 12 randomised, controlled trials in psoriasis, PsA, and Crohn’s disease in which 5,884 patients received ustekinumab showed that major adverse cardiovascular events, malignancies, and death were rare.10 Moreover, preliminary analysis of real-world data in psoriasis, from PSOLAR (a global psoriasis register of 12,095 patients), shows that the drug with the longest survival (a surrogate marker of efficacy, safety, and tolerability) is ustekinumab, ahead of adalimumab, infliximab, and etanercept (Figure 1).11 According to the PSOLAR data, cumulative incidence rates of the adverse events of special interest (malignancy, major adverse cardiovascular events, serious infections, and mortality) are relatively low with ustekinumab; the rate of serious infections is particularly low.12
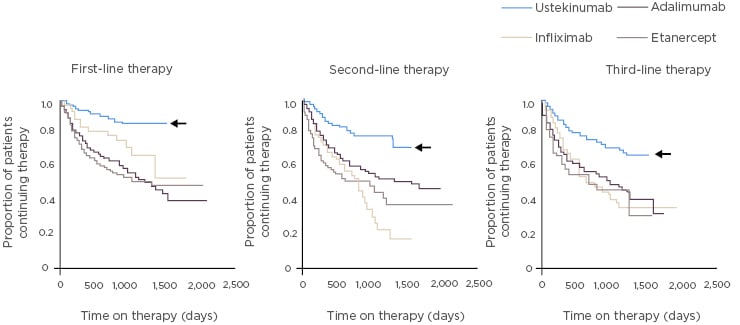
Figure 1: Drug survival in patients with psoriasis in PSOLAR.11
PSOriasis Longitudinal Assessment and Registry (PSOLAR) study: global psoriasis register of 12,095 patients, with ~4,000 patients initiating a new biologic therapy.
In PSUMMIT 1 and 2, ustekinumab was associated with significant improvements in enthesitis and physical function measures.5,13,14 Treatment with ustekinumab (45 mg or 90 mg) significantly reduced the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) at 24 weeks (p=0.0019–<0.0001 versus placebo),5 and this improvement in enthesitis was associated with improvement in physical function as measured by the Health Assessment Questionnaire Disability Index (HAQ-DI) and 36-item Short-Form Health Survey (SF-36).14 Furthermore, in the head-to-head ECLIPSA study, significantly more patients treated with ustekinumab (73.9%) achieved a score of 0 on the Spondyloarthritis Research Consortium of Canada (SPARCC) scale at 6 months than TNFi-treated patients (41.7%; p=0.018; primary endpoint);15 in the same study, reductions in tender joint count and swollen joint count were similar between the two treatment groups.15
Real-world treatment with ustekinumab in PsA has previously only been investigated in several small observational studies.16-18 The PsABio study is a prospective observational cohort of patients with PsA from eight European countries who were starting ustekinumab or a TNFi as a first, second, or third-line biologic therapy (N=992);19-21 6-month, PsABio data presented at this EULAR Congress show that only 7.6% of patients on ustekinumab and 10.2% on a TNFi stopped or switched biologics within 6 months.19 Ustekinumab and TNFi performed similarly well in achieving remission or low disease activity as assessed by measures including clinical Disease Activity in Psoriatic Arthritis (cDAPSA) remission, and minimal disease activity.20 The mean swollen joint count in 66 joints was reduced from baseline by 4.3 (ustekinumab) and 4.5 (TNFi); and the tender joint count in 68 joints was reduced from baseline by 6.4 (ustekinumab) or 6.7 (TNFi).22
Dr Siebert concluded that IL-12/23 pathway inhibition with ustekinumab is well tolerated and effective across a range of domains, with low or minimal disease activity achieved and sustained in a significant proportion of patients with psoriatic disease. Nonetheless, PsA remains a difficult disease to treat; there are still unmet needs (e.g., lack of head-to-head trials, uncertainty about best treatment strategy for an individual). Experience of targeted therapies and real-world data from large cohorts will help advance understanding of the disease and lead to better patient outcomes.
Focus on the IL-23 Pathway
Professor Georg Schett
IL-23, a dimer of p19 and p40 subunits, was identified in a relatively recent search for IL-6 family cytokines in sequence databases in 2000.23 IL-23 is produced by antigen-presenting cells, mainly dendritic cells (DC); the skin contains numerous DCs and is, therefore, a major location of IL-23 production. DC also produce IL-12 (a dimer of p35 and p40); these cytokines together trigger the activation of T cells.23,24 The key function of IL-23 is to induce T cell proliferation.23
Whether DC predominantly produce p19 or p35 (and, therefore, IL-23 or IL-12, respectively) depends upon their cellular and molecular environment; p19 production can be linked to autoinflammatory disease.25 For example, in a murine model of multiple sclerosis, increased p19 expression induces experimental autoimmune encephalomyelitis and triggers T helper Type 17 (Th17) cell differentiation, thus exacerbating autoimmune-triggered inflammation.25
IL-23 triggers psoriasis and entheseal inflammation in humans;4,26 analysis of skin from patients with psoriasis shows that p19 and p40 are upregulated in lesional skin.27 This finding suggests that IL-23 is produced in situ in inflamed skin.27 Importantly, if p19 is blocked in skin, hyper-proliferation, skin thickness, and skin infiltration by CD4+ and CD8+ T cells are significantly reduced (all p<0.01–<0.05 versus placebo), but resident Langerhans cells are unaffected.28 Targeting p19, therefore, has an anti-inflammatory effect achieved by downregulation of a network of key pathogenic immune pathways in psoriasis and PsA such as T cell chemotaxis (e.g., the chemokine CCL20), neutrophil chemotaxis (e.g., the chemokine CXCL8), IL-17 pathway activation (e.g., the IL-17 target gene lipocalin 2) and innate immune activation (e.g., the alarmin S100A7, also known as psoriasin).28 Interestingly, preclinical studies have shown that the p19 subunit also dimerises with an Epstein–Barr virus-induced protein to form IL-39,29,30 but there is no evidence yet that IL-39 has a biological function in humans.30,31
As mentioned, IL-23 also has a key role in enthesitis.32 When triggered by mechanical stress, disturbed barrier function, or infections, IL-23 (with prostaglandin E2) stimulates the accumulation of IL-17-producing γδ T cells.33 Production of IL-17, TNFα, and IL-22 by γδ T cells, along with Type 3 innate lymphoid cells, instigates enthesitis.34 Enthesitis-driven PsA is highly sensitive to IL-12/23 inhibition by ustekinumab, as shown by reductions from baseline in SPARCC score, MASES, and Leeds Enthesitis Index (LEI) score in the ECLIPSA study.15
In autoimmunity, IL-23 controls the pathogenicity of antibodies by regulating their glycosylation (sialylation) and, thus, their effector function, essentially ‘unlocking’ them for use.35 Under conditions of high sialylation of IgG, autoantibodies are in a non-inflammatory or ‘locked’ state, and asymptomatic autoimmunity results in mice.34 Under conditions of low sialylation of IgG, inflammatory autoantibodies are ‘unlocked’ and autoimmune disease results.35 In a IL-23 knockout mouse model of collagen-induced arthritis, the production of key effector cytokines of inflammation (TNFα, IL-6, and CXCL1) was impaired when the IgG was in its sialylated (‘locked’) state, suggesting that autoimmune inflammation is at least partly controlled by IL-23.35 Interestingly, IL-23 deficiency mitigates experimental lupus in mice.36
Prof Schett concluded that IL-23, produced by DC and other innate immune cells, polarises T cells to a Th17 phenotype, thereby influencing downstream adaptive immune responses. The role of IL-23 in skin and entheseal inflammation, T/B-cell interaction, and auto-antibody effector function suggests therapeutic value of IL-23-targeting in autoimmune and autoinflammatory disease.
Targeting IL-23: What Could This Mean in Practice?
Professor Peter Taylor
The efficacy of biologic and small-molecule treatments in PsA has been evaluated in multiple clinical trials in patients with predominantly skin and/or joint involvement. All licensed drugs have significantly better efficacy in terms of joint outcomes, as assessed by rates of 20% improvement in American College of Rheumatology criteria (ACR20) at Week 24, versus placebo (drug classes: TNFi, IL-17 inhibitors, and ustekinumab; ACR20 rates range: 36.6–63.8%; p value range: ≤0.001–<0.0001).5,37-44
The IL-17 family (IL-17A, IL-17A/F, and IL-17F), along with IL-25, signal via the IL-17 receptor.45,46 Approved drugs, ixekizumab and secukinumab, block IL-17A; brodalumab blocks the IL-17 receptor so potentially affects IL-17A, IL-17F, and IL-25; bimekizumab (in development) blocks IL-17A/F.46 Clinical trial data on these drugs are crucial to understanding how clinical findings relate to the underlying pathophysiology of psoriatic disease.
Phase III trials of secukinumab (FUTURE 2) and ixekizumab (SPIRIT P1) show that in patients with PsA, IL-17A inhibition significantly improved joint and skin outcomes, as assessed by ACR and Psoriasis Area and Severity Index (PASI) 75 response rates at Week 24 (all p<0.001 versus placebo).39,44 Additionally, in a Phase II trial of brodalumab in patients with PsA, improvement in joint outcomes at Week 12 (p<0.05 versus placebo) was sustained to Week 108.47,48 Conversely, IL-17A/F blockade has been reported to lead to impaired immunity to fungal and extracellular bacterial infections,49 and in some studies in Crohn’s disease, gut symptoms were exacerbated by treatment with IL-17 inhibitors.50-52
Targeting upstream of IL-17 with ustekinumab enables reduction in T-helper cell activity (Th1 and Th17) and subsequent IL-17 expression.53,54 In patients with PsA naïve to biologics (PSUMMIT 1), ustekinumab significantly improved ACR response rates versus placebo at Week 24 (p≤0.0001 for ACR20, 50, and 70).5 Moreover, in patients already exposed to a biologic (i.e., TNFi, PSUMMIT 2), ACR response rates were slightly lower than observed in PSUMMIT 1 but significantly better with ustekinumab than placebo (ACR20, p<0.001; ACR50, p<0.05).5,7
IL-23 is targeted by risankizumab, tildrakizumab, and guselkumab.54 Blocking IL-23 alone is expected to block intracellular signals from Th17 but not Th1 cells.53,54 In patients with rheumatoid arthritis (RA), blocking IL-12/23 (ustekinumab) or IL-23 (guselkumab) in a Phase II study did not achieve the primary endpoint (ACR20 at Week 28),55 suggesting that RA and PsA are distinct diseases that involve different pathways in their aetiology; however, results from Phase II trials examining IL-23 inhibition in PsA have demonstrated, and importantly retained, efficacy.56,57 For example, patients with PsA receiving one single dose of the anti-IL-23A antibody risankizumab at baseline had significantly better ACR20 response rates at Week 16 versus placebo (p<0.05).56
Retained efficacy, even after switching from placebo, is evident with guselkumab in PsA.57 In a Phase II study, patients with PsA were randomised to guselkumab or placebo then crossed over to guselkumab at Week 24.57 Treatment with guselkumab resulted in significant improvement in joint and skin outcomes at Week 24 as assessed by ACR (Figure 2) and PASI.57 At Week 44, ACR responses in patients who switched to guselkumab caught up with results in the group who initiated guselkumab at baseline (Figure 2).57 Rates of patients reaching these endpoints increased with time, and at all response levels the benefit was sustained for several months after treatment.57
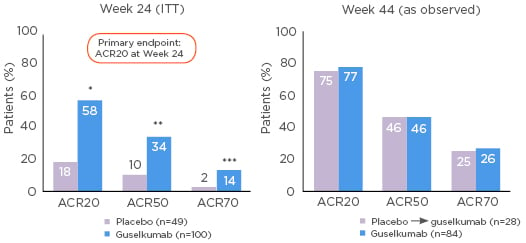
Figure 2: Guselkumab treatment led to significant improvements in ACR outcomes versus placebo at Week 24 in patients with psoriatic arthritis. Patients switching from placebo to guselkumab at Week 24 had similar outcomes to the guselkumab group at Week 44.57
*p<0.0001; **p=0.0021; ***p=0.023 versus placebo. ACR: American College of Rheumatology criteria; ITT: intention to treat population.
Treatment with guselkumab also resulted in significant improvements in physical function at Week 24 (HAQ-DI score; p=0.0002 versus placebo) and resolution of enthesitis (p=0.0120) and dactylitis (p<0.0010).57 Notably, patients had significant improvement in mental as well as physical aspects of quality of life (as measured by the SF-36), perhaps reflecting the dramatic improvement in their skin symptoms.57 Guselkumab was generally well-tolerated through to Week 56, and serious adverse events were rare.56 No injection site reactions were reported.57
Prof Taylor concluded that the IL-23 and IL-17 pathways are promising therapeutic targets in PsA. The availability of targeted therapies and advances in engineering techniques has facilitated dissection of pathobiological disease components to provide insights into the ‘immunotaxonomy’ of rheumatic diseases, and some understanding of the clinical correlates of that information. Further advances in precision medicine and biomarkers to inform treatment decisions will change disease management, but until then, optimal therapy for patients will depend on certain comorbidities (e.g., inflammatory bowel disease [IBD], uveitis) and whether they predominantly have skin or joint symptoms. The benefit:risk ratio of emerging therapies is not yet clear, and we await emerging Phase III data.
Experience of Targeting IL-23 in Dermatology
Professor Lluís Puig
In psoriasis, an autoimmune process that depends on IL-23 leads to differentiation of naïve T cells to Th17 cells, promoting the production of IL-17.58 Subsequent activation of keratinocytes produce a variety of chemotactic factors in a feed-forward mechanism that sustains the inflammatory process in psoriatic skin.58 This process can be controlled, but tissue resident ‘memory cells’ that express IL-23 receptor can be rapidly reactivated to reproduce psoriatic lesions.58 Thus, IL-23 is the ‘master switch’ for the inflammatory process underlying psoriasis.
Prof Puig suggested that upstream targeting (i.e., IL-23) may be more convenient, allowing less frequent dosing and less need for induction treatment. Therapeutic longevity (i.e., maintenance of response over time) is typical of IL-23 inhibition but less so with IL-17 blockade. Furthermore, the causal relationship between IL-17 inhibition and exacerbations of IBD and/or candidiasis is inconclusive.
In VOYAGE 1 and 2, guselkumab treatment was efficacious and high levels of response (PASI 90 and PASI 100) were maintained for up to 156 weeks in patients with psoriasis, even after switching from placebo (at Week 16) or TNFi (at Week 28) to guselkumab.59–61 VOYAGE 1 was a three-arm trial in which patients were randomised to guselkumab, adalimumab, or placebo then crossed over to guselkumab at Week 16.59 VOYAGE 2 was similar in design to VOYAGE 1; however, at Week 24, patients were re-randomised, depending on their response: responders (PASI 90) were randomised to (continue) guselkumab or placebo then crossed over to guselkumab upon loss of ≥50% of their Week-28 PASI response.60
In both studies, approximately 70% of patients with psoriasis achieved PASI 90 with guselkumab at Week 16, and by Week 24 rates were significantly higher for guselkumab than adalimumab (p<0.010).59,60 PASI 100 rates increased with time,59,60 reaching approximately 50% for guselkumab-treated patients at Week 48.59 Importantly, patients switching to guselkumab from placebo at Week 16 showed significantly higher PASI 90 and 100 rates than for adalimumab by Week 48 (p<0.001).59 Additionally, guselkumab maintained response rates to Week 156, with approximately 80% and 50% of patients achieving PASI 90 and 100, respectively.61
Patients who did not achieve PASI 90 on adalimumab achieved and maintained PASI 90 after the switch to guselkumab.62 Of the patients who did not reach PASI 90 at Week 52 and 28 in VOYAGE 1 and 2, respectively, after switching to guselkumab, >70% had reached PASI 90 and >40% PASI 100 at Week 100. These findings show that guselkumab is effective not only as a first-line therapy but also as a second-line therapy after adalimumab.62,63
Guselkumab is also effective after a suboptimal response to ustekinumab, as demonstrated in NAVIGATE.64 Patients received open-label ustekinumab and, depending on Investigator’s Global Assessment score (IGA), were randomised to guselkumab or ustekinumab (IGA≥2); those with IGA 0 or 1 continued ustekinumab.64 Between Weeks 20 and 52, improvements in IGA ≥0/1, PASI 75, 90, and 100 response rates were higher in patients who switched to guselkumab than in those who continued ustekinumab.64
Therapeutic longevity is shown with guselkumab in patients who, after achieving PASI 90, were randomised to placebo in the VOYAGE 2 study.60,65 At 6 months after their last injection of guselkumab, approximately 50% of patients maintained PASI 90 and 30% maintained PASI 100 after 5.5 months.60 Of the patients who lost >50.0% of their initial response after withdrawal of guselkumab, 87.6% regained PASI 90 by Week 28 after restarting guselkumab.65
In the head-to-head ECLIPSE study, patients with psoriasis (excluding those with a history of IBD) were randomised to guselkumab or secukinumab.66 By Week 48 there was an approximately 14.0% higher rate of PASI 90 response to guselkumab than to secukinumab (p<0.001; Figure 3).66 The safety of the two drugs through Week 56 was comparable but generally better with guselkumab than with secukinumab.66 There was a slightly higher rate of infections with secukinumab (64.8%, versus guselkumab: 58.6%), and IBD developed in 3 of the 511 patients in the secukinumab group (versus none in the guselkumab group).66
Figure 3: Guselkumab treatment led to significantly higher response rates of PASI 90 (with 95% CI) versus secukinumab through Week 48 in patients with psoriasis.66
Non-responder imputation was used for missing data. CI: confidence interval; PASI: Psoriasis Area and Severity Index.
In conclusion, Prof Puig suggested that guselkumab is suitable for first-line treatment in patients with psoriasis, for second-line treatment after failure of adalimumab, and for patients with an insufficient response to ustekinumab. Responses to guselkumab are persistent over time and highly sustainable, and guselkumab has been superior to secukinumab (demonstrated by PASI 90 response rate at Week 48) in long-term studies. Guselkumab is efficacious, convenient (injected at Weeks 0 and 4, then every 8 weeks), and well tolerated (comparable with ustekinumab).
Panel Discussion and Concluding Remarks
The faculty responded to a variety of questions during the panel discussion. The audience members were interested in the treatment decision-making process and asked the panel about the ideal patient for ustekinumab treatment. Prof Puig suggested that patients with extensive skin disease, as well as enthesitis, dactylitis, and peripheral arthritis, would be suitable for ustekinumab, but it has failed to show efficacy in patients with axial spondyloarthritis. Dr Siebert added that it is important to consider safety in patients with multiple comorbidities; ustekinumab’s safety profile makes it a suitable treatment for patients with skin disease and enthesitis who also experience complications due to comorbidities.
Prof Puig pointed out that clinical trial findings regarding entheseal disease are dependent on patient-reported outcomes, and although there are promising data on IL-23 blockade in enthesitis, the apparent lack of effect in axial disease is puzzling. Prof Schett explained that this may be due to different environments in different tissues (e.g., skin, spine, and peripheral entheseal tissue), with differences in IL-23 producing cells. It needs to be considered that spinal disease is probably not just one disease, but differences between axial spondyloarthritis and PsA need to be considered. In support of this concept, he emphasised that a post-hoc analysis of PSUMMIT data showed that patients with PsA and concomitant axial disease responded to treatment,67 further evidence that there may be differences in axial disease patterns between classic ankylosing spondylitis and axial involvement in PsA. Dr Siebert agreed that there may be some IL-23-independent production of IL-17, and head-to-head studies are needed to better understand PsA.
The audience asked about the low malignancy rates seen with targeted therapies, and whether these differ from those in the US Food and Drug Administration (FDA) database. Dr Siebert called for caution when comparing real-world and clinical trial data, due to differences in patient populations. Prof Puig noted that no increase in risk of malignancies has been noted with targeted therapies in patients with psoriasis, except for one epidemiologic study, showing an increased risk of non-melanoma skin cancer in patients treated with anti-TNF agents;68 these patients are likely to have been exposed to coal tar, photochemotherapy, and cyclosporin, which increase their risk.
When asked about switching therapies after inadequate response, and the optimal treatment sequences in difficult-to-treat patients, the faculty responded that the current best approach is to switch to a treatment with a different target. Patients who seem to develop antidrug antibodies might be switched to one of the less immunogenic treatments. The faculty noted that switching decisions are based on whether the relapse is predominantly skin, peripheral joint or axial disease, with a need for more finely tuned therapy in PsA than in RA.
Prof Taylor commented that in some countries biosimilar use is encouraged to achieve cost savings, though patients with comorbidities may benefit more from other options. Dr Siebert added that patients with PsA are generally more risk-averse and less tolerant of side effects than patients with arthritis. Prof Schett agreed that, with the availability of distinct immune interventions and the possibility to tailor patient-specific treatments, it would be a pity if choices would be merely dictated by costs.
According to Prof Puig, it should be possible to prescribe treatments other than anti-TNF as first-line therapy, especially for those patients in whom infection might decompensate their pre-existing comorbidities. He emphasised the importance of finding the immunological mechanism underlying the skin disease, enthesitis, or joint disease, and eventually making treatment decisions based on this information. Prof Taylor, who closed the discussion, noted that rheumatologists and dermatologists will still face several challenges in their treatment decision processes in the future.