Meeting Summary
The symposium discussed mechanisms of interleukin (IL)-6 blockade for the treatment and management of patients with rheumatoid arthritis (RA). Prof Smolen provided a clinical update of the latest efficacy and safety data on various anti-IL-6 drugs, including sirukumab. He noted that all anti-IL-6 drugs were efficacious in treating physical and mental symptoms of RA. When the efficacy of anti-IL-6 antibodies was compared between drugs, targeting the IL-6 ligand was similar to targeting its receptor. Prof Pitzalis described the pathophysiology of IL-6 in RA and the reason for targeting IL-6. Lastly, Prof Choy outlined the importance of measuring patient-reported outcomes to monitor symptom improvement and evaluate the impact of IL-6 on mental functioning. Because IL-6 modulates the hypothalamic pituitary axis, fatigue and depression are common in patients with RA. Evidence suggests that the inhibition of IL-6 activity reduces symptoms of fatigue and depression in patients with RA, and that improvement in mental health occurs independently, rather than as a consequence of improvement in physical functioning.
Welcome and Introduction
Professor Costantino Pitzalis
The objective of the symposium was to explain the mechanism behind IL-6 dysfunction in RA pathophysiology, review novel RA therapies and their effect on structural damage and patient-reported outcomes, and consider the use of IL-6 inhibitors in patients with RA to manage psychological symptoms.
The Role of Interleukin-6 in Rheumatoid Arthritis Pathophysiology and Structural Damage
Professor Costantino Pitzalis
IL-6 is a keystone cytokine responsible for the pathogenesis of RA,1 a chronic systemic inflammatory condition driven by synovitis, which causes joint damage,2,3 bone erosion, and joint narrowing due to cartilage damage. IL-6 mediates effects both at the local and systemic level,2,3 evident by the high levels of IL-6 in both the synovial fluid and serum of patients with the disease.2
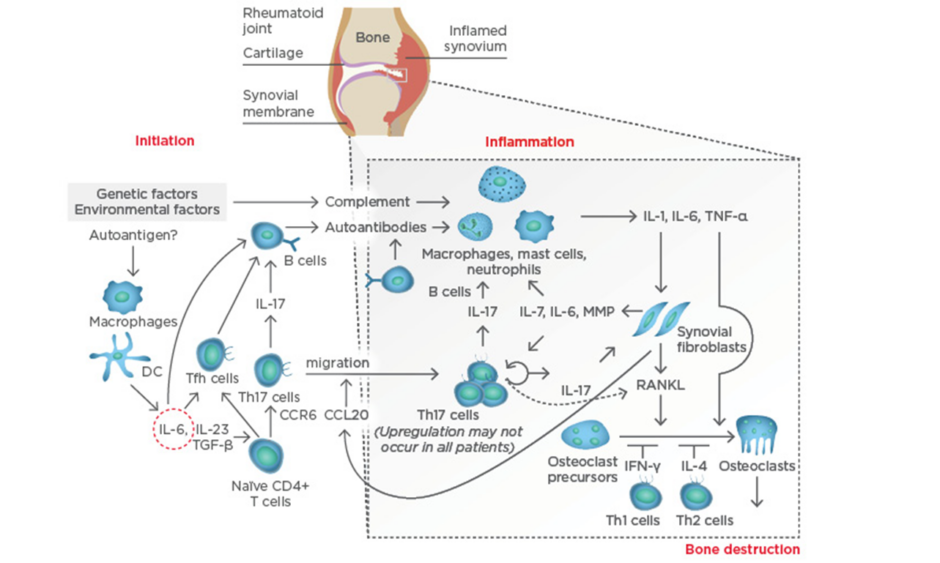
At the systemic level, IL-6 plays a key role in the aberrant immune responses leading to autoimmunity in RA. IL-6 drives T follicular helper cells and the differentiation and maturation of B-cells into plasmablasts and plasma cells. IL-6, together with transforming growth factor-beta (TGF-β) and IL-23, also drives the activation and differentiation of T helper (Th)17 cells, which are highly pathogenic. Thus, IL-6 contributes to the activation of the immune system that, following stimulation by still not fully determined arthritogenic antigens, leads to breach of tolerance (particularly to citrullinated antigens) and the production of autoantibodies; the presence of these antibodies is a disease indicator, and they can circulate for years unnoticed without causing any symptoms until a ‘second hit’ reaches the synovial membrane in the joint. At the joint level, a complex immunological cascade is activated in which IL-6 again mediates critical aspects of the immune system, including the activation of macrophages, the differentiation of specific T cell and B cell subsets, and the subsequent activation of synovial fibroblasts and the osteoclasts that lead to joint damage (Figure 1).
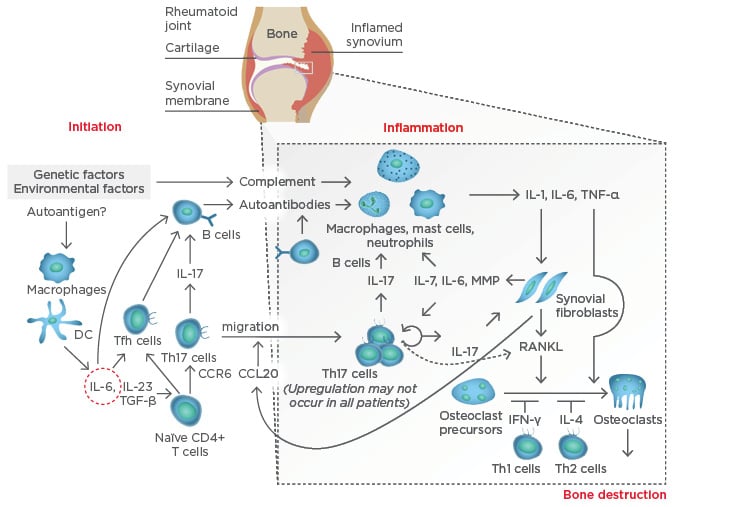
Figure 1: Pathogenesis of rheumatoid arthritis.
CCL20: chemokine ligand 20; CCR6: chemokine receptor type 6; DC: dendritic cell; IFN-γ: interferon gamma; IL: interleukin; MMP: matrix metalloproteinases; RANKL: receptor activator of nuclear factor kappa-B ligand; Tfh: T follicular helper; TGF-β: transforming growth factor-beta; Th: T helper; TNF-α: tumour necrosis factor-alpha. Adapted from Komatsu et al.4
IL-6 has widespread effects due to its extensive production by multiple cells and the broad expression of IL-6 receptors.5 Importantly, the IL-6 receptor α chain is primarily based in haematopoietic cells while the β chain, the glycoprotein 130 (GP130), is widely expressed also on non-haematopoietic and stromal cells. For example, IL-6 mediates chronic synovitis and pannus formation, and together with the RANK ligand, triggers osteoclastogenic pathways1 contributing to subchondral bone erosions and joint damage. Evidence has shown that sirukumab (an antibody that binds to the IL-6 ligand) is effective in preventing structural damage progression. Markers of bone destruction (e.g. C1M, C3M, C4M) were reduced, while markers associated with cartilage regeneration and bone turnover were increased (e.g. β-isomerised C-terminal telopeptides of Type I collagen (CTX-1) and the N-terminal-Mid fragment of osteocalcin).6
Downstream effects outside the joint include IL-6 targeting the liver and inducing acute phase reactants (e.g. C-reactive protein [CRP], an important measure of disease activity in patients with RA, and hepcidin), which interferes with iron homeostasis leading to hypoferraemia and anaemia commonly seen in chronic diseases.7
Another example is the inflammation mediated by IL-6 that can increase cardiovascular disease risk.7 Systemic inflammation tends to exacerbate prior cardiovascular risk factors, which is why inflammation in RA is linked to accelerated atherosclerosis.8 Elevated IL-6 levels are also associated with increased mortality in patients with acute coronary syndrome and increased risk of myocardial infarction in healthy men, regardless of whether RA inflammation was present.8
Lastly, the influence of IL-6 on the hypothalamic–pituitary axis has been associated with fatigue and mood changes.9 High levels of IL-6 in the blood are associated with high levels of IL-6 in the brain, since it can cross the blood–brain barrier.10,11 Following inflammation, not only can high levels of IL-6 be found within the brain, but also widespread GP130 expression.12 Furthermore, proinflammatory cytokines, such as tumour necrosis factor-alpha (TNF-α) and IL-1 (and also toll-like receptors), can induce de novo production of IL-6 from microglia, astrocytes, endothelial receptors, and certain types of neurons.12
IL-6, together with TNF-α and IL-1, activate a homeostatic pathway that begins with the release of corticotropin-releasing hormone and arginine vasopressin in the paraventricular nuclei of the brain. Corticotropin is then released and activates the adrenal release of cortisol, which then attempts to turn off the expression of proinflammatory cytokines. IL-6 has an effect of the locus coeruleus (the principal site for synthesis of noradrenaline), which may lead to fatigue and low mood state (Figure 2).9,13
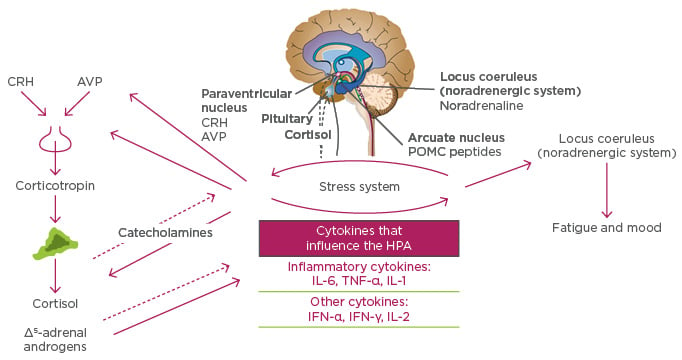
Figure 2: Cytokines affect fatigue and mood in rheumatoid arthritis by influencing the hypothalamic pituitary adrenal axis.
AVP: arginine vasopressin; CRH: corticotropin-releasing hormone; HPA: hypothalamic-pituitary-adrenal axis; IFN-γ: interferon-gamma; IL: interleukin; POMC: proopiomelanocortin; TNF-α: tumour necrosis factor-alpha. Adapted from Chrousos9 and Tsigos and Chrousos.13
Interleukin-6 Blockade: A Clinical Update
Professor Josef S. Smolen
IL-6 is a ubiquitous cytokine in the immune system. The majority of IL-6 receptors are membrane-bound3,14 and cannot transduce signals on their own. IL-6 transduction occurs via multimerisation of the membrane and soluble-bound forms of IL-6 receptors (cleaved from membrane-bound receptors by ADAM17) and GP130, and subsequent activation of the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathways, which leads to cell survival, cell growth, cell proliferation, cell-cycle progression, increased protein synthesis, and drug resistance;15 all of which are key to inflammation (Figure 3).
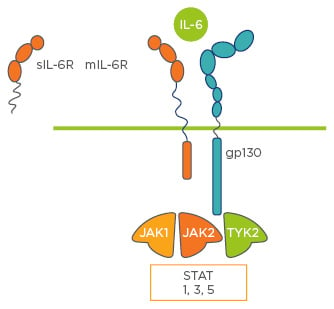
Figure 3: The interleukin-6 pathway.
IL: interleukin; gp130: glycoprotein 130; JAK: Janus kinase; mIL-6R: membrane interleukin-6 receptor; sIL-6R: soluble interleukin-6 receptor; STAT: signal transducers and activators of transcription; TYK: tyrosine kinase 2.
The IL-6 ligand, its receptors, and GP130, are all potential targets for the treatment of RA. Antibodies developed as potential treatments bind to different targets in the IL-6 signalling pathway; some antibodies bind to the IL-6 receptor, others to the IL-6 ligand, and a few can inhibit IL-6 signal transduction. This section outlines evidence that anti-IL-6 antibodies are efficacious treatments for patients with the disease. Generally, response rates decrease in patients with increasing history of treatment and stronger medication (e.g. anti-TNF-α drugs), regardless of whether they are undergoing active therapy or placebo, because they are more resistant to treatment. However, there is evidence to suggest that all anti-IL-6 drugs mentioned in this summary are efficacious in managing RA regardless of prior treatment history. Furthermore, they have similar efficacy and safety profiles regardless of their mode of action. Therefore, this section summarises data on the efficacy and safety of four anti-IL-6 antibodies: tocilizumab, sarilumab, clazakizumab, and sirukumab.
Tocilizumab
Tocilizumab is an antibody that can bind to both membrane and soluble-bound forms of IL-6 receptors. Results from various trials have shown a statistically significant greater rate of American College of Rheumatology response criteria (ACR)50 and ACR70 (percent scores in relation to changes in RA symptoms) responses in patients who received tocilizumab, regardless of prior medication (methotrexate, disease-modifying anti-rheumatic drugs [DMARD], or anti-TNF-α). For example, data from a pooled analyses of trials (AMBITION,16 LITHE,17 OPTION,18 and RADIATE19) matched prior findings: ACR70 responses were reported in 28% of methotrexate-naïve responders, 19% of DMARD-insufficient responders, and 12% of anti-TNF-α-insufficient responders.
Results have also shown radiographic benefit, reduction in disease progression, and improvement in quality of life. The LITHE trial showed that patients who received tocilizumab combined with methotrexate had higher Genant-modified Sharp scores (measure of erosion and joint space narrowing) at Week 52, with a statistically significant difference of 0.3 in each dose group (4 mg/kg and 8 mg/kg) compared to 1.1 in the placebo group. More specifically, both doses resulted in a lower value in both erosion and joint space narrowing numerical scores, which implies that tocilizumab is associated with reduced bony and cartilaginous destruction.17 The OPTION trial18 showed that both dose groups of tocilizumab combined with methotrexate showed a greater reduction in Health Assessment Questionnaire (HAQ) score (measure of health-related quality of life) than patients who took methotrexate as monotherapy in the control group, which did not reach the minimal clinically important difference of -0.22 before Week 12.
Further to this result, the SURPRISE trial20 showed that, in patients who did not respond to methotrexate treatment, the rate of DAS28 remission (measure of disease activity reduction) was higher when tocilizumab was combined with methotrexate than when tocilizumab was used as monotherapy. The rate of disease activity score in 28 joints (DAS28) remission at Week 24 was 69.6% in the group that received combination therapy, compared to 55.0% in the group that received tocilizumab as monotherapy, a statistically significant difference. This implies that tocilizumab works best in combination with methotrexate. This finding is supported by the FUNCTION trial,21 which showed that the number of ACR50 and ACR70 responses were higher in the group that received the combination therapy compared with the two groups that received either of them as monotherapy.
Sarilumab
Sarilumab is an anti-IL-6 receptor antibody like tocilizumab, so they were both expected to have a similar efficacy profile. Results from the MOBILITY trial22 showed that methotrexate-insufficient responders exhibited higher rates of ACR20, ACR50, and ACR70 responses and lower progression of erosion and joint space narrowing (measured by the modified Total Sharp score) when they were treated with sarilumab combined with methotrexate, compared with those who were given placebo combined with methotrexate. The incidence of ACR70 response was 25% for 200 mg of sarilumab, 20% for 150 mg, and 7% for placebo, which is in line with data from methotrexate-naïve participants in tocilizumab. In another study,23 anti-TNF-insufficient responders (i.e. patients who are more resistant to treatment effects than methotrexate-insufficient responders) who were given sarilumab combined with conventional synthetic DMARD had ACR70 response rates of 16.3% for sarilumab 200 mg, 19.9% for 150 mg, and 7.2% in placebo, which is again in line with tocilizumab results. In conclusion, sarilumab has a similar efficacy profile to tocilizumab, potentially due to their common mode of action.
Clazakizumab
Clazakizumab is an antibody that binds to the IL-6 ligand, so although it has a different mechanism than the previous two drugs, results show similar efficacy to anti-IL-6 receptor antibodies. A Phase IIb trial24 showed greater ACR20 response rates at Week 12 in patients who received clazakizumab combined with methotrexate, compared with those who received clazakizumab as monotherapy, but both types of therapy showed better response rates than those in the placebo plus methotrexate group. Adalimumab (an anti-TNF drug) combined with methotrexate was just as effective a treatment as 25 mg of clazakizumab combined with methotrexate, and both produced the highest response rates out of all the groups (76.3% and 78.0%, respectively). Therefore, clazakizumab as a monotherapy has less efficacy compared with clazakizumab combined with methotrexate and an anti-TNF drug combined with methotrexate.
Sirukumab
Sirukumab is another antibody that binds to the IL-6 ligand. In a Phase II trial,25 the ACR50 response rate at Week 12 was higher across all doses of sirukumab compared to the placebo group, with 100 mg of sirukumab taken every 2 weeks showing a statistically significant difference of 26.7% of participants compared to 3.3% of participants in the placebo group. A Phase III programme comprising several studies and this section will focus on the key findings of the SIRROUND-D trial and the SIRROUND-T trial.
The SIRROUND-D trial consisted of 104 weeks of therapy with 16 weeks follow-up for participants who failed to respond to DMARD. The primary endpoints were ACR20 response rate at Week 16 and change in van der Heijde Sharp score from baseline to Week 52, which is a measure of structural damage. For both primary endpoints, both doses of sirukumab showed statistically superior results compared to the placebo group; in fact, both doses were associated with an 80% reduction in progression rate of structural damage compared to the placebo group (0.5 versus 3.7, respectively).25 The statistical significance also applied to ACR50 response rate, physical function,26 and mean change in fatigue and quality of life (measured with SF-36) at Week 24.27 In terms of physical function, the placebo group did not meet the minimal clinical significant difference (0.22) and both groups of sirukumab exceeded it two-fold.
The SIRROUND-T trial,28 which consisted of 52 weeks of therapy, with 16 weeks of follow-up for participants that failed to respond to TNF inhibitors, showed the same results as SIRROUND-D. ACR20 and ACR50 response rates, physical function, and quality of life at Week 16 were significantly higher in both sirukumab groups compared to the placebo group and, in terms of physical function, the placebo group did not meet the minimal clinical significant difference, whereas both groups of sirukumab exceeded it. In a pre-specified subgroup analysis, a similar response rate was shown regardless of prior therapy and the number of drugs that patients failed to respond to. Patients who failed to respond to more than one had lower response rates than those who failed to respond to only one, regardless of whether they were undergoing active treatment or placebo, but the ACR20 response rate at Week 16 was still 40% for those who had received two prior TNF inhibitors, and 36–39% for those who failed three. Lastly, a post-hoc analysis showed statistically higher ACR20 response rates at Week 16 even among participants who failed TNF inhibitors and tocilizumab, which implies that IL-6 ligand inhibitors work even if IL-6 receptor inhibitors fail.
Safety data on sirukumab gathered from pooled randomised clinical trial data28 show similar results to what have been reported in previous studies on the topic. Serious infections are more likely with sirukumab than with placebo, but not more likely than other similar agents. This is because IL-6, as well as having proinflammatory side effects, also has homoeostatic effects on the immune system. For example, IL-6 is a key growth factor in the bone marrow, so inhibition of IL-6 may lead to neutropaenia, which is associated with rare but serious infection.
In summary, sirukumab, an anti-IL-6 ligand antibody, showed efficacy regarding clinical, functional, and structural outcomes, as well as physical function and quality of life. Its safety profile is within expectations based on prior data of IL-6 receptor inhibitors (primarily tocilizumab).
The Biology Behind Patient-Reported Outcomes in Rheumatoid Arthritis
Professor Ernest Choy
Current regulations (e.g. from the U.S. Food and Drug Administration [FDA]) require patient-reported outcomes to be measured as key endpoints in clinical trials.29 A systematic review carried out at John Hopkins University, Baltimore, Maryland, USA,30 showed that the top three patient-reported domains that were measured by randomised clinical trials (RCT) were physical function (65 RCT; measured by HAQ-Disability Index [HAQ-DI), pain (30 RCT; measured by pain 100 mm Visual Analogue Scale [VAS] for pain), and patient global VAS (27 RCT; measured by VAS 100 mm). Fatigue and health-related quality of life were also frequently measured.
Fatigue
Fatigue is measured frequently across studies because it is a common symptom across many chronic physical and mental illnesses. A cross-sectional study of 238 patients with RA31 showed that the mean fatigue score was 49 mm on the VAS; 54% of patients scored >50 mm and 84% of patients had clinically relevant fatigue defined by a fatigue score of ≥20 mm. Fatigue score was correlated with disease activity (r=0.48; measured by DAS28), degree of pain (r=0.68), and HAQ score (r=0.51), which may be due to reduced ability to carry out daily activities with increased fatigue.
A conceptual framework created by OMERACT of the manifestation of fatigue in patients with RA32 showed that the cause of fatigue in this disease is multi-factorial and there are components of fatigue that may not necessarily be related to RA, such as components related to general inflammation or components more broadly related to cognitive, personal, or socio-environmental factors. Another finding was that patients are more prone to fatigue if they suffer from anxiety or depression. Evidence from the Cochrane meta-analysis comparing both TNF inhibitors and non-TNF agents shows that blocking inflammation reduces fatigue;33 thus, one significant component that drives fatigue in many patients with RA is inflammation.
Depression
Depression is commonly reported in people with RA. A German study measured the prevalence of depression in patients with RA by using four different measures of depression to capture its different aspects, including mood changes and low mood levels.34 Consequently, prevalence of depression ranged from 18.5–49.5%, and all prevalence rates were higher than the general population. Furthermore, all four measures showed a correlation between depression scores and the DAS28 score (p<0.001), implying a correlation between depression and the level of inflammation. A Japanese study supports this correlation when using the Beck Depression Inventory to measure mood changes by showing that depression was correlated with CRP levels.35
Depression is very common in sufferers of chronic inflammatory diseases in general, and blocking inflammation can improve depression independently rather than as a result of improved physical functioning. A meta-analysis showed that TNF inhibitors were associated with reduced depression compared with placebo. More specifically, TNF inhibitors were more effective in reducing depression than standard DMARD.36 A meta-analysis of four studies showed that sirukumab was linked with improved prevalent depressed mood and anhedonia independently of improvement in physical function.37 Therefore, the difference in patient-reported mood level remains significant even when the data control for improvement in disease activity.
It is possible that patients with endogenous depression may actually be suffering from an inflammatory disease. A meta-analysis supports this hypothesis, with findings that people with endogenous depression had higher levels of IL-6 levels and TNF-α levels.38 It is also possible that blocking cytokines can reduce depressive symptoms in patients with endogenous depression. The SIRROUND-D trial reported that the physical component score from a 36-item short form survey (SF-36 [a widely used measure of health-related quality of life]) was correlated with changes in the HAQ score. This was reflected by improvement in the mental component score from SF-36 as well; in fact, all four aspects of the score reached or exceeded the minimally important clinical difference for each of these domains.39 The same result was shown in the SIRROUND-T trial.28 This concludes that both doses of sirukumab improved fatigue in tandem with physical functioning compared with placebo, with no difference in improvement between doses. Another finding was that the mental health aspect of the mental component score improved independently of change in vitality, role-emotional, and social functioning, which supports the hypothesis that inhibiting IL-6 activity can improve depression independently of improvement in physical functioning.
Biological Explanation
The reason for the close relationship between IL-6 activity and mental functioning is that IL-6 can pass through the blood–brain barrier10,11 and influence activity in the hypothalamic–pituitary axis, which regulates stress responses and other bodily responses such as the immune system. Via mechanisms not yet understood, IL-6 within the hypothalamic–pituitary axis can lead to fatigue and mood changes.9,13 In summary, evidence from patient-reported outcomes suggests that cytokines are implicated in the pathogenesis of fatigue and depression in RA, and that inhibiting cytokines can reduce fatigue and depression.