Abstract
A treat-to-target (T2T) strategy is a treatment plan in which the clinician treats the patient aggressively enough to reach and maintain explicitly specified and sequentially measured goals. To apply a T2T strategy, some conditions should be met. First, a proactive, clear endpoint should be used and a threshold should be defined. Second, a choice between several effective therapies must be available. Third, the endpoint should be supported by findings from randomised controlled trials supporting early aggressive treatment. Fourth, the strategy should be cost-effective. Finally, it needs to be acceptable by the stakeholders.
The objective of this review was to verify if the conditions for applying the T2T strategy were met in psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), using a narrative review.
Based on the currently available literature, the conditions for applying the T2T in PsA and axSpA were partially met. First, proactive outcome measures are available; however, there is no clear consensus regarding the optimal one. Second, there is a reasonable choice of approved therapies for both diseases. Third, additional randomised controlled trials demonstrating the effectiveness of a T2T approach are still needed. Fourth, cost-effectiveness studies are needed and should include patients from different healthcare systems. Fifth, the implementation of T2T recommendations in routine care and the adherence to its application in clinical practice should be promoted.
In summary, preliminary data suggest that T2T might be beneficial to patients with PsA and axSpA. However, further studies are needed to meet all the criteria before strongly advocating for T2T strategies.
INTRODUCTION
A treat-to-target (T2T) strategy is a treatment plan in which the clinician treats the patient aggressively enough to reach and maintain explicitly specified and sequentially measured goals, such as remission, low disease activity, or absence of disability.1,2
The T2T concept was broadly used in non-rheumatological diseases, such as hypertension,3 diabetes,4 and dyslipidaemia5 and has shown to improve important clinical outcomes such as preventing cardiovascular events, diabetic retinopathy, and even ultimate outcomes such as mortality.
In rheumatology, T2T was applied successfully in rheumatoid arthritis,6 in gout,7 and in systemic lupus erythematosus,8 with clear target cut-offs in disease activity scores and serum urate levels, respectively, correlating with the prevention of radiographic damage.9,10
Specific recommendations for T2T in rheumatology were first developed in rheumatoid arthritis in 2010,6 followed by recommendations for spondyloarthritis (SpA; including ankylosing spondylitis [AS] and psoriatic arthritis [PsA]) in 2012, which were later updated in 2017.11 They were adopted, although conditionally, in the international management recommendations for PsA12,13 and axial spondyloarthritis (axSpA).14,15 In addition to the goal of optimising the quality of life by decreasing symptoms, inflammation, and structural damage, the T2T recommendations in SpA must face an additional challenge: they must address extra-musculoskeletal manifestations (EMMs) as possible targets, which makes the ‘target’ a much more heterogeneous and complicated one.
To apply a T2T strategy, some general conditions should be met (Table 1). First, a proactive, clear endpoint, which is the treatment aim, should be used in a specific target algorithm and a threshold should be defined. Second, a choice between several effective therapies that allow the clinical goal to be achieved must be available. Third, the endpoint should be supported by findings from randomised controlled trials (RCTs) suggesting that early aggressive treatment approaches are advantageous. Fourth, the proposed strategy should be cost-effective. Fifth, it needs to be acceptable by the stakeholders.16
Although the T2T approach is well established in RA, its relevance and applicability in PsA and axSpA are still debated.
OBJECTIVE
The objective of this review was to verify if the conditions for applying the T2T strategy were met in PsA and in axSpA.
METHODS
Using the key words “treat to target”, “rheumatology”, and “spondyloarthritis” in PubMed, the authors conducted this narrative review. First, the authors identified the conditions that are required to apply a T2T strategy and summarised them in five questions.
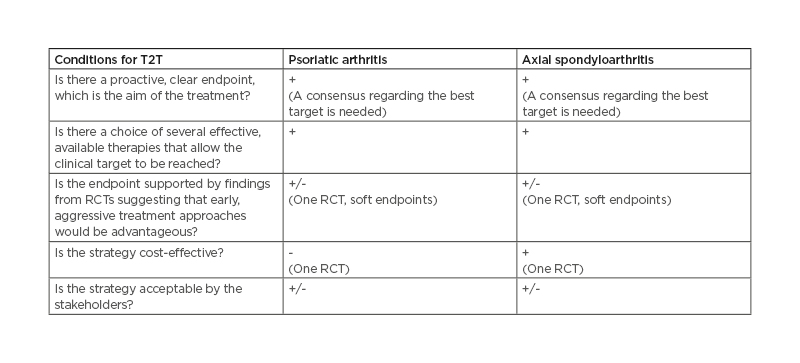
Table 1: Summary of conditions needed to apply a T2T strategy in psoriatic arthritis and axial spondyloarthritis.
RCT: randomised controlled trial; T2T: treat-to-target.
Then, the responses to these five questions were sought for PsA and axSpA, respectively.
Is there a proactive, clear endpoint, which is the aim of the treatment?
A ‘good’ endpoint or target must be easily measurable in clinical practice, be validated in patients with PsA and axSpA, respectively, and reflect clinical outcomes that are important to both patients and physicians. The choice of the target should be a shared decision between the patient and the rheumatologist, considering all relevant situational factors. Treatment, once started, should be monitored to investigate if the endpoint is reached. The endpoint should be used in a specific target algorithm, and a threshold should be defined. Since both diseases, particularly PsA, are very heterogeneous, encompassing arthritis, enthesitis, dactylitis, and/or psoriasis in the same patient, finding the optimal target is challenging.
The targets can be soft, reversible outcomes (i.e., disease activity score or inflammatory markers) or hard, irreversible outcomes (i.e., radiographic damage or disability).11 In most cases, soft endpoints correlate with the hard outcomes while being easier to obtain, thus often serving as surrogate measures for the hard, more relevant outcomes.17-21
Moreover, the clinician must keep in mind the EMMs and take them into account when facing a specific clinical situation.
Is there a choice of several effective, available therapies that allow the clinical target to be reached?
A treatment is usually considered effective when its value has been demonstrated by high-quality RCTs. For the current analysis, the authors included the therapies that are recommended by international rheumatology bodies such as the American College of Rheumatology (ACR)/National Psoriasis Foundation (NPF),13 the European Alliance of Associations for Rheumatology (EULAR),12,14 the Assessment of Spondyloarthritis International Association (ASAS),14 and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).22
Any therapy whose efficacy was demonstrated in a recent RCT published after issuing these recommendations was also evaluated. Non-pharmacological treatments were evaluated, as well as a mean to treatment optimisation.
Is the endpoint supported by findings from RCTs suggesting that early aggressive treatment approaches would be advantageous?
RCTs comparing the T2T strategy to the standard of care in PsA and axSpA, respectively, were reviewed.
Is the strategy cost-effective?
Cost-effectiveness studies were analysed from the identified RCTs.
Is the strategy acceptable by the stakeholders?
Acceptability was first evaluated by checking if the T2T strategy was adopted in the PsA and the axSpA recommendations (ACR, ASAS, GRAPPA, and EULAR). Second, studies regarding the implementation of T2T in clinical practice and its related perceptions by patients and by healthcare providers (HCP) were analysed.
Finally, strategies for implementing and adopting T2T in clinical practice were discussed, and the unmet needs and areas for future research were identified.
RESULTS
Psoriatic Arthritis
Is there a proactive, clear endpoint, which is the aim of the treatment ?
Many composite measures exist and can be potential candidates for use as a proactive endpoint.11,23 These measures include ACR outcome measure, Arithmetic mean Desirability Function (AMDF) composite score, Composite Psoriatic Disease Activity Index (CPDAI), Disease Activity in Psoriatic Arthritis (DAPSA), Disease Activity Score 28 joints (DAS-28:), Group for Research and Assessment of Psoriasis and Psoriatic Arthritis Composite Index (GRACE), Minimal Disease Activity (MDA), Psoriatic Arthritis Disease Activity Score (PASDAS), Psoriatic Arthritis Response Criteria (PsARC), Routine Assessment of Patient Index Data 3 (RAPID3), and Very Low Disease Activity (VLDA) (Table 2). All of these measures include joint counts: tender (TJC) and swollen (SJC). Many scores include the patient’s and/or the physician’s global assessment; some of them include inflammatory markers, skin outcomes, or evaluation of dactylitis, enthesitis, or axial involvement.
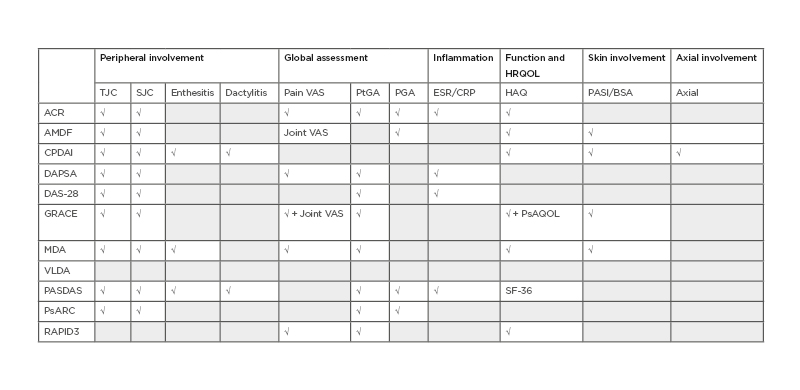
Table 2: Composite measures used in psoriatic arthritis.
Grey boxes indicate that the composite measure does not include the corresponding item.
ACR: American College of Rheumatology; AMDF: Arithmetic mean Desirability Function composite score; BSA: body surface area; CRP: C-reactive protein; CPDAI: Composite Psoriatic Disease Activity Index; DAPSA: Disease Activity in Psoriatic Arthritis; DAS-28: Disease Activity Score 28 joints; ESR: erythrocyte sedimentation rate; GRACE: Group for Research and Assessment of Psoriasis and Psoriatic Arthritis Composite Index; HAQ: Health Assessment Questionnaire; HRQOL: Health-Related Quality of Life; MDA: minimal disease activity; PASDAS: Psoriatic Arthritis Disease Activity Score; PASI: Psoriasis Area Severity Index; PGA: Physician Global Assessment; PsARC: Psoriatic Arthritis Response Criteria; PtGA: Patient Global Assessment; RAPID3: Routine Assessment of Patient Index Data 3; SJC: swollen joint count; TJC: tender joint count; VAS: visual analogue scale; VLDA: very low disease activity.
Overall, according to two systematic literature reviews, there is an important heterogeneity regarding the composite outcome measure used in PsA studies; therefore, a consensus in this area is a clear unmet need.24,25 In a GRAPPA meeting including 26 rheumatologists, dermatologists, and patient research partners, the panel could not reach a consensus regarding a continuous measure of disease activity.23
A comparison of remission and low disease activity states with DAPSA, MDA, and VLDA in a clinical trial setting in patients with PsA concluded that both DAPSA and MDA composite measures (Table 3) can be used for evaluation of the status and treatment response utilising a T2T approach and can improve patient health-related outcomes. These two measures were also the ones that were preferred in the 2017 T2T recommendations.11 Likewise, in a clinical trial setting of the study using DAPSA and MDA in secukinumab-treated patients with PsA, both composite measures were useful for evaluation of the status and treatment response utilising a T2T approach.26
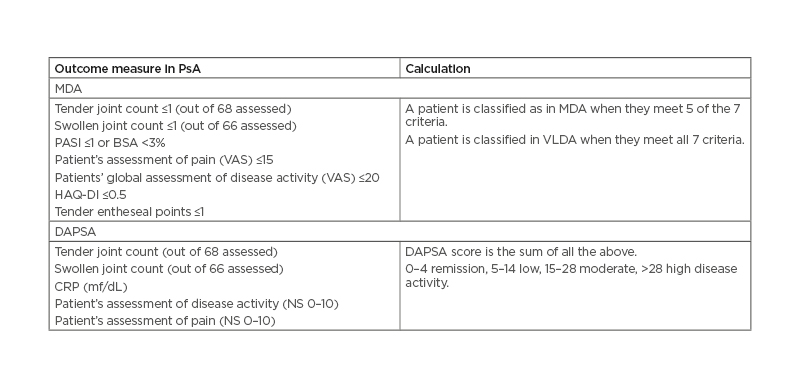
Table 3: MDA criteria and DAPSA are the most recommended outcome measures in psoriatic arthritis.
BSA: body surface area; DAPSA: Disease Activity in Psoriatic Arthritis; HAQ-DI: Health Assessment Questionnaire Disability Index; MDA: minimal disease activity; NS: numerical scale; PASI: Psoriasis Area Severity Index; VAS: visual analogue scale; VLDA: very low disease activity.
On the one hand, the DAPSA was initially developed for reactive arthritis.27 It has been validated for use in PsA, where it showed correlational, discriminatory, and criterion validity; furthermore, it was sensitive to change in trials and observational studies alike and has shown a good correlation with ultrasound-assessed synovitis.28,29 Schoels et al.30 provided criteria for disease activity states and treatment response, which showed good performance in clinical trials and observational data. It is a simple measure and specifically measures peripheral arthritis without the inclusion of any other domains. Therefore, a separate assessment of skin disease and potentially other domains should be mandated alongside the DAPSA score to ensure a full assessment of PsA disease activity. DAPSA use was recommended by the T2T 201711 and the ACR 201813 PsA management recommendations.
On the other hand, the MDA encompasses several disease domains (joint counts, global assessment, skin assessment, HAQ, enthesitis) and is increasingly accepted. The MDA criteria were specifically developed with the idea of investigating the benefits of T2T in PsA31 and were validated in PsA in 2010 and used as a key outcome measure in the main T2T trial in PsA.32 MDA use was recommended by the GRAPPA 2015,22 the T2T 2017,11 and the ACR 201812 management recommendations. In a recent analysis, Mease et al.33 compared the disease control thresholds in the Corrona PsA/axSpA registry. They confirmed the previously described notion that MDA and VLDA were the most stringent disease activity measures and resulted in overall lower disease activity in multiple key domains compared to patients who met clinical DAPSA, Patient Acceptable Symptom State (PASS), Patient Global Assessment of Arthritis (PtGA), and Patient Global Assessment of Arthritis and Psoriasis (PtGA PA) thresholds.25 Therefore, they encouraged the rheumatologists to use MDA/VLDA to assess disease control in patients with PsA.
Furthermore, since PsA is a very heterogeneous disease, recent head-to-head trials have used combined outcomes to reflect the complexity of the disease, including targets in the joint (ACR) and the skin measurements (Psoriasis Area and Severity Index; PASI) that must be reached simultaneously.34,35 This approach has helped distinguish some patients’ profiles where a specific therapeutic class can be effective. Moreover, patients simultaneously achieving ACR 50% improvement (ACR50) and PASI 100% improvement (PASI100) had consistently better improvements in other T2T outcomes, including MDA and VLDA.36
All the mentioned scores are only surrogates for the hard endpoints, which mainly include radiographic damage and long-term disability and represent the ultimate goal of any therapy in PsA.11 However, these hard endpoints are slow to achieve and their inclusion in a T2T strategy, where timely interventions are needed, remains challenging.
Beyond the discussed endpoints, EMMs are also essential to address. The prevalence of common EMMs in PsA is 90% for psoriasis, 3–7% for inflammatory bowel diseases, and 1–3% for uveitis.37,38 They should be identified and managed in a multidisciplinary setting as their presence may significantly impact the treatment decision.
Finally, the choice of the target of the disease activity should take comorbidities, patient factors, and drug-related risks into account.11
Is there a choice of several effective available therapies that allow the clinical target to be reached?
The choice of therapies for PsA has tremendously increased during the last decade. Several effective therapies are now approved, and many others are under study (Table 4).
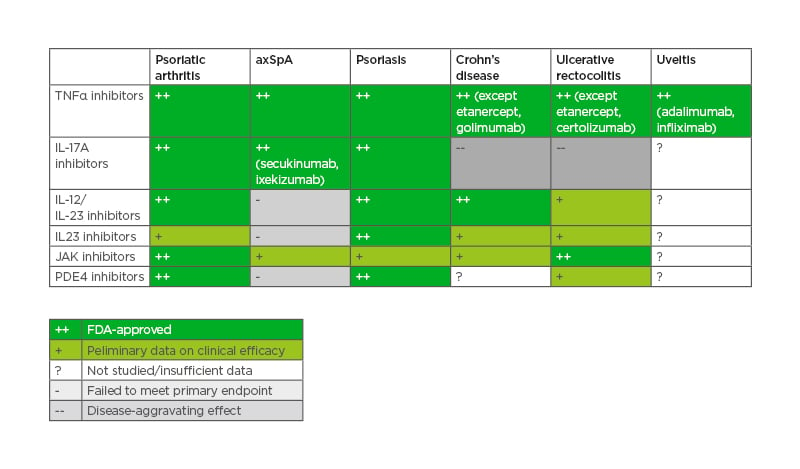
Table 4: Effective therapies in psoriatic arthritis, axial spondyloarthritis, and extra-musculoskeletal manifestations.
axSpA: axial spondyloarthritis; FDA: U.S. Food and Drug Administration; PDE4: phosphodiesterase 4.
According to the EULAR, ACR/NPF, and GRAPPA recommendations,12,13,22 conventional synthetic disease-modifying drugs (cs-DMARDs, usually methotrexate) can be used after the failure of non-steroidal anti-inflammatory drugs (NSAIDs), except when axial disease and/or enthesitis are predominant. After the failure of cs-DMARDs, the biologic therapies (anti-TNFα, anti-IL-12/IL-23, anti-IL-17A) and the small molecules (JAK inhibitors, phosphodiesterase type 4 inhibitors) are recommended. They should be prioritised according to the main domain involved and several treatment algorithms were proposed by the international, regional, and local recommendations. More recent data regarding anti-IL23-p19 seem to be very promising, expanding the therapeutic armamentarium furthermore.39-41
Is the endpoint supported by findings from RCTs suggesting that early aggressive treatment approaches would be advantageous?
Evidence of the effectiveness of the T2T strategy was assessed in PsA in a key trial, the TICOPA study,32 published in 2015.
TICOPA was an open-label study conducted in the UK and included 206 patients who were DMARD-naïve and had a PsA of short duration (<2 years). The study duration was 48 weeks.
The patients were randomised to either standard therapy with 3-monthly evaluations with no strict guidance about the treatment decisions, or tight control (TC) with 4-weekly evaluations and step-up therapy (starting with methotrexate and stepping-up to adalimumab) if MDA was not reached. At Week 12, MDA was achieved in 24% of the TC group. The proportion of patients reaching ACR20, ACR50, ACR70, and PASI75 was significantly higher in the TC group. Moreover, a significantly greater improvement was observed for patient-reported outcomes in the TC arm. Regarding the treatments, the use of biologics was much higher in the TC group, and this group had a much higher incidence of adverse events.
Regarding radiographic progression, although it was numerically lower in the TC group, it did not reach statistical significance at Week 48. It was argued that the included population had mild disease with low baseline radiographic scores and consequently a low risk for radiographic progression.42
Other studies where therapy was altered based on achieving a target were conducted with anti-TNF clinical trials.24 They included a plan in the study protocol for an escalation of treatment if pre-specified targets were not met, labeled as an ‘early escape’ arm where patients at a set time point (12 or 16 weeks), still in the double-blind portion of the study, could be re-randomised to potentially increase therapy if a particular reduction in their disease activity was not met. The target used in these studies was the reduction in the number of tender and swollen joints, which is a questionable endpoint. Moreover, these studies were not investigating the impact of T2T in a robust comparison against standard care.
Therefore, there is a clear need for additional RCTs that investigate the value of T2T strategies in PsA. Moreover, evidence on the effect of these strategies on the long-term outcomes, namely radiographic damage, function, and health-related quality of life, is essential.
Is the strategy cost-effective?
An analysis from the TICOPA study32 from the perspective of the UK National Health System (NHS) found that when this strategy was applied in a nation-wide sample, the incremental cost-effective ratio was 54,000 GBP (70,200 USD) per quality-adjusted life year (QALY), which exceeded the threshold allowable by the NHS and drove the authors to conclude that T2T strategy in PsA was not cost-effective.43 The analysis did not incorporate indirect costs to patients, such as productivity loss; incorporating such costs likely would make tight control even less favourable due to its expense.
Is the T2T strategy acceptable by the stakeholders?
The T2T concept was adopted by the 2018 GRAPPA recommendations for the use of composite measures and treatment targets in PsA,23 the ACR 2018,13 and the EULAR 2020 PsA management recommendations.12 The ACR gave only a conditional recommendation for the use of the T2T strategy over not following a T2T strategy, and stated that one might consider not using a T2T strategy in patients in whom there are concerns related to increased adverse events, costs of therapy, and patient burden of medications associated with tighter control. The latest EULAR recommendations updated in 2020 rephrased their T2T recommendation. They specified that the target should be remission or low disease activity (instead of minimal disease activity), while acknowledging the difficulty of defining remission and suggesting using the abrogation of inflammation as an indicator of remission. They gave this recommendation a Grade A, with a high level of agreement (9.4).
When considering whether T2T is applied in practice, studies showed that it was adopted by only a minority of patients.44 From the patients’ perspective, they may have adapted to their disease and became reluctant to change if they feel ‘OK’ even if they still have some disease activity. Also, they might disagree with the physician’s measure of their disease activity. Furthermore, some patients who are required to make out-of-pocket contributions to healthcare might be unwilling to visit their rheumatologist more frequently.1
Regarding the HCPs, a GRAPPA survey showed that 56% reported that they do T2T in clinical practice.23 Also, a qualitative study of clinicians’ perspectives identified the barriers to implementation of T2T in PsA using interviews with rheumatologists and other healthcare professionals:45 individual motivation to change clinical practice, lack of consensus on what to measure, what is achievable with limited resources, and mandatory versus voluntary pressures to change. Moreover, T2T requires frequent visits and the use of standardised outcomes measures, which may be challenging for rheumatologists with busy practices.1
Axial Spondyloarthritis
Is there a proactive, clear endpoint, which is the aim of the treatment?
For the first time in the history of SpA research, evidence has been accrued to suggest the value of ‘targeting disease activity’ because disease activity leads to new syndesmophytes in patients with axSpA.17,18
Many endpoints were proposed in T2T strategies in axSpA: from markers of disease activity (C-reactive protein [CRP], Bath Ankylosing Spondylitis Activity Index [BASDAI],46 Ankylosing Spondylitis Disease Activity Score [ASDAS],47-49 Assessment of Spondyloarthritis International Association [ASAS] Remission); 50 to markers of structural progression, disability (ASAS Health Index; ASAS-HI),51 as well as comorbidities (smoking cessation, NSAID intake, hypertension, diabetes).
Also, these include EMM and sequelae of the long-standing disease, such as cardiovascular disease and osteoporosis.
The two most-used target measures for axSpA in clinical practice are the BASDAI and the ASDAS (Table 5).52
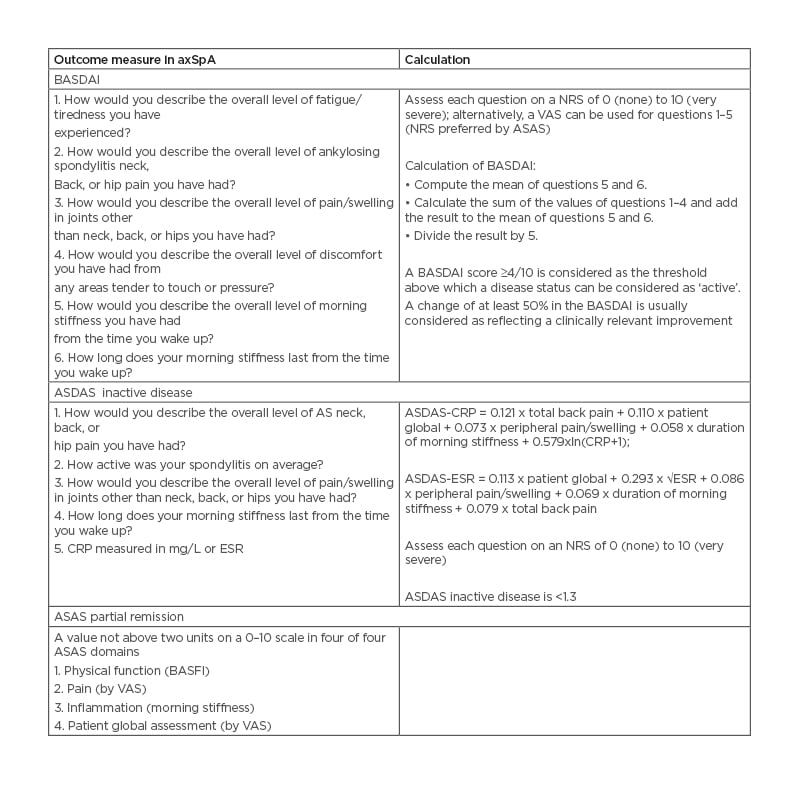
Table 5: Bath Ankylosing Spondylitis Activity Index (BASDAI) and Ankylosing Spondylitis Disease Activity Score (ASDAS) are the most recommended outcome measures in axial spondyloarthritis.
ASAS: Assessments of Spondyloarthritis International Society; ASDAS: Ankylosing Spondylitis Disease Activity Score; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; BASFI: Bath Ankylosing Spondylitis Functional Index; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; NRS: numerical rating scale; VAS: visual analogue scale.
The ASDAS was developed to attempt to overcome some of the limitations of the BASDAI. Indeed, due to the subjectivity of the items included on the BASDAI, there is often discordance between patient and clinician assessments of the disease activity.53 ASDAS includes some questions from the BASDAI as well as patient and physical global assessments and laboratory measures (either the CRP or the erythrocyte sedimentation rate [ESR]). However, the ASDAS has some limitations: it does not incorporate other objective measures of inflammation, such as those found on imaging. ASDAS has validated thresholds for disease activity categories, whereas BASDAI does not, and it is the preferred measure in axSpA according to T2T international task force recommendations.11
Recent studies showed that achievement of an inactive disease status (ASDAS 1.3) while on treatment with anti-TNFα resulted in almost complete radiographic spinal progression inhibition during the following 2-year radiographic interval.16,19 Other anti-TNFα trials showed that 15–35% of patients reach ASDAS-Inactive Disease (ASAS-ID).20,21 ASAS partial remission can be used, but its main limitation is that it relies partly on the Bath Ankylosing Spondylitis Functional Index (BASFI), and a patient with irreversible structural damage may be unable to fulfill ASAS partial remission criteria.54 ASDAS low disease activity can also be a therapeutic target,11,16 but it is still debated because of the lack of data.52
In response to the lack of a definition of AS disease severity, ASAS developed an instrument based on the International Classification of Functioning, Disability, and Health (ICF) model of function and health, the ASAS-HI.51 A value ≥12.0 serves as the cut-off between poor and moderate health, whereas a value <5.0 is the cut-off between good and moderate health. The ASAS-HI serves as the primary outcome measure in a recent T2T trial in axSpA.55
As for the structural damage evaluation, it could be performed by sacroiliac and spine radiographs and also by MRI. Some have proposed that imaging could be used in patients in clinical remission in whom tapering of biologics is considered.2 However, this suggestion was refuted by Smolen et al.11 because there are no data justifying the use of imaging in follow-up yet, and it is not feasible to perform MRI repeatedly in axSpA.
Less-conventional outcomes, such as smoking cessation, NSAID use, and cardiovascular disease, require a long follow-up time and are therefore not easy to assess in RCTs but can be assessed in prospective cohort studies.2 However, such information would be relevant to understand the effects of treatment on long-term complications of axSpA and to optimise the T2T approach, especially in cases where access to different biotherapies is not simple.
As mentioned earlier, the choice of the target and of the disease activity should take comorbidities, patient factors, and drug-related risks into account.11
As for the EMMs, according to a meta-analysis, the pooled lifetime prevalence of common EMMs in patients with axSpA were 26% for uveitis, 9% for psoriasis, and 7% for inflammatory bowel disease.56 They should also be evaluated using a multidisciplinary approach.
Is there a choice of several effective, available therapies that allow the clinical target to be reached?
As with PsA, the choice of therapies for axSpa has increased considerably over the past decade and several effective therapies are now approved (Table 3).
According to the EULAR-ASAS14 and the ACR15 recommendations, after NSAIDs failure, the biologic therapies (anti-TNFα, anti-IL-17A) are recommended. They should be prioritised according to the main domain involved, and several treatment algorithms were proposed by the international recommendations. Data from several retrospective observational studies analysis suggested that anti-TNF therapy can delay the radiographic progression in the long term.19,57,58 Additionally, tofacitinib, a JAK inhibitor, has shown promising results, adding to the armamentarium of the treatment of axSpA.59
Is the endpoint supported by findings from RCTs suggesting that early aggressive treatment approaches would be advantageous?
To date, there have been two T2T trials in axSpA.
The STRIKE study60 was a German RCT of patients with axSpA meeting the ASAS axSpA criteria and having been symptomatic for <5 years, who were randomised to T2T versus usual care. In the T2T arm they were assessed monthly, and the protocol involved starting with an NSAID and escalating to adalimumab. The primary outcome was ASDAS inactive disease (ASDAS-ID) at 32 weeks. Unfortunately, this trial was stopped due to slow recruitment.52
TICOSPA55 is a European pragmatic, prospective, cluster-randomised controlled trial of patients with axSpA, comparing tight control with monthly assessments to usual care for one year. The primary outcome is change in the ASAS-HI over 1 year. Secondary outcome measures include ASDAS, BASDAI, quality of life, and resource utilisation. The strategy was pre-specifed by the scientific committee based on current axSpA recommendations and aiming at a target of ASDAS <2.1, with visits every 4 weeks. The treatment decisions in usual care arms were at the rheumatologists’ discretion, with visits every 12 weeks. One hundred and sixty patients were included (80 in TC and 80 in usual care). The mean age was 37.9 (±11.0) years with a disease duration of 3.7 (±6.2) years. 51.2% were males. Radiographic damage of the sacroiliac joints, an (ever) positive MRI sacroiliitis, and HLA-B27+ were seen in 46.9%, 81.9%, and 75.0% of patients, respectively. Mean ASDAS at inclusion was 3.0 (±0.7) and mean ASAS-HI was 8.6 (±3.7). Although 47.3% versus 36.1% patients in the TC and usual care arms achieved an improvement in ASAS-HI at the 1-year visit, which was considered clinically relevant, the difference was not statistically significant. Across all other outcomes, a trend was observed in favor of the TC arm. The number of biological DMARDs was significantly higher in the T2T arm (56.2% versus 27.2%). The number of infections was comparable in both groups (15 versus 16 in the TC and usual care arms, respectively).
Is the strategy cost-effective?
To date, there is one cost-effectiveness analysis of T2T strategies in axSpA. Indeed, an analysis from the TICOSPA study found that when this strategy was applied, the T2T strategy was cost-effective with an incremental cost-effective ratio of 19,430 EUR. From a societal perspective, T2T resulted in an additional 0.04 QALY and saved 265 EUR when compared to usual care and a 67% probability of being cost-effective at a cost-effectiveness threshold of 20,000 EUR per QALY.55
Is the strategy acceptable by the stakeholders?
Smolen et al.11 indicated that with T2T strategies, all the options were acceptable; namely, to be left as they had been initially constructed, amended, deleted, or expanded in number and/or changed in sequence. But the lack of evidence available led some societies, such as ACR, not to recommended T2T strategies in axSpA.
Indeed, the ACR 2019 conditionally recommended the regular-interval use and monitoring of a validated AS disease activity measure, and conditionally recommended regular-interval use and monitoring of CRP concentrations or ESR over usual care without regular CRP or ESR monitoring.
For adults with active AS or non-radiographic axSpA, they conditionally recommended against using a T2T strategy using a target of ASDAS <1.3 (or 2.1) over a treatment strategy based on physician assessment. For patients and providers, the panel felt that more convincing evidence of benefit should be present before approving this change in practice. Their rationale was that they feared that choosing a specific target would lead to rapid cycling of all currently available treatments in some patients. That said, they emphasised the importance of having targets in the management of patients.61
The EULAR/ASAS 2016 recommendations recommended that a target should be defined and documented, but, unlike the T2T international task force and the ACR guidelines,11,61 refrained from mentioning the content of such target. This target may change depending on the phase of the disease and the treatments already used previously.
DISCUSSION
Based on the currently available literature, the conditions for applying the T2T in PsA and axSpA are partially met.
First, proactive outcome measures are available, however, there is no clear consensus regarding the choice of the optimal measure. Using a universal target allows for better comparability between the clinical trials. Moreover, soft endpoints should be validated against a gold-standard hard endpoint (such as long-term disability, quality of life measures, and/or radiographic damage). DAPSA and MDA in PsA and ASDAS in axSpA should be correlated with radiographic scores and long-term measures of disability to properly estimate the effect of a treatment strategy on the general burden of the disease, respectively. Acceptable cut-off scores for soft outcomes, in relationship to these hard outcomes, should be adopted on larger scales. Furthermore, the inclusion of radiologic measures and clinical activity related to EMMs in T2T studies should be discussed but may require longer follow-up studies.
Second, there is a choice of several available therapies for both diseases, and the treatment armamentarium is constantly increasing.
Third, additional RCTs demonstrating the effectiveness of a T2T approach in providing an advantage over the standard care are still needed. Researchers should continue to evaluate whether current therapeutic tools are sufficient to reach the proposed targets and investigate the benefit from the active implementation of non-pharmacological treatments in the T2T strategies.
Fourth, cost-effectiveness studies are needed and should include patients from different healthcare systems. Also, the inclusion of non-pharmacological treatments, particularly in settings of low economic resources, should be considered.
Fifth, the implementation of T2T recommendations in routine care and the adherence to its application in clinical practice should be promoted.62,63 Financial constraints, staff shortages, patients’ reluctance, and high clinic demands are among the reasons for implementation difficulties. Many methods are available to implement T2T in clinical practice. They rank from the least to the most effective: education, rules and policies, reminders and checklists, simplification and standardisation, and forcing functions.2 T2T may also be successfully implemented if rheumatologists are required to enter detailed data into registries.64 Moreover, the role of non-physician HCPs such as rheumatology nurses in the implementation of T2T in clinical practice should be evaluated, following very successful experience in diabetes, hypertension, and hyperlipidaemia.65,66 Furthermore, the role of electronic health records that prompt rheumatologists about escalation/de-escalation opportunities and capture their medical decision-making could allow the development of refined T2T care strategies and deserves further evaluation.67
CONCLUSION
In summary, T2T is an emerging management strategy in PsA and axSpA. Preliminary data suggest that a T2T approach might be beneficial to patients with PsA and axSpA. However, further studies are needed to meet all the required criteria before strongly advocating for T2T strategies in clinical practice.







