Abstract
Background: Steroid-induced hyperglycemia is a common side effect of steroid therapy, such as prednisone, used in the treatment of various conditions, including rheumatoid arthritis. Elevated blood glucose levels characterize this metabolic disturbance(steroid-induced hyperglycemia), which can pose challenges in patient management, particularly in those without prior diabetes history.
Aim: The authors present the case of a 58-year-old female with rheumatoid arthritis who developed hyperglycemia after initiating prednisone therapy. It aimed to illustrate the clinical course, management, and outcomes of steroid-induced hyperglycemia in this patient.
Clinical details: The patient, with a history of rheumatoid arthritis, received a short-term course of prednisone due to worsening symptoms. Within a week, she developed symptoms of hyperglycemia despite no prior history of diabetes. Laboratory tests confirmed elevated blood glucose levels and a glycated hemoglobin of 8.5%. Her blood glucose remained elevated, despite dietary modifications and oral hypoglycemic agents, requiring insulin therapy.
Outcomes: Insulin therapy initiation with a basal-bolus regimen led to gradually recovering blood glucose levels. Regular monitoring and adjustments resulted in stable fasting and postprandial glucose levels within target ranges. The patient reported symptom improvement and increased energy levels.
Conclusion: This case highlights the importance of vigilant monitoring and management of steroid-induced hyperglycemia in patients receiving steroid therapy, even for short durations. Collaborative efforts between rheumatology, endocrinology teams, and clinical pharmacists were crucial in ensuring optimal disease control while minimizing long-term steroid use. Individualized treatment plans and patient education are essential for optimizing outcomes and reducing risks associated with steroid-induced hyperglycemia.
Key Points
1. Steroid-induced hyperglycemia is a frequent side effect of steroid therapy, caused by mechanisms such as
insulin resistance and increased hepatic glucose production, and can occur even with short-term steroid use.
2. The case of a 58-year-old female with rheumatoid arthritis illustrates how prednisone led to significant hyperglycemia, requiring insulin therapy after oral hypoglycemic agents were insufficient. Management strategies included close glucose monitoring, dietary modifications, and insulin therapy, with collaborative care from rheumatology, endocrinology, and clinical pharmacy teams.
3. The case highlights the importance of early recognition, patient education, and individualized treatment plans to manage steroid-induced hyperglycemia and minimize long-term steroid use.
BACKGROUND
Steroids are widely used in various medical conditions, including rheumatoid arthritis, asthma, and inflammatory bowel disease. They possess potent anti-inflammatory effects by suppressing the immune system and reducing inflammation. However, one of the significant side effects associated with the use of steroids, such as prednisone, is steroid-induced hyperglycemia.1
Steroid-induced hyperglycemia is a common metabolic disturbance determined by increased blood glucose levels.2 The mechanisms underlying steroid-induced hyperglycemia are multifactorial. Steroids can impair insulin sensitivity and glucose uptake in peripheral tissues, leading to insulin resistance. Additionally, they stimulate hepatic gluconeogenesis, resulting in increased hepatic glucose production. These mechanisms contribute to elevated blood glucose levels in patients receiving steroids, leading to steroid-induced hyperglycemia.3
The incidence of steroid-induced hyperglycemia varies depending on the dose and duration of steroid therapy. Higher doses and prolonged use of steroids are associated with an increased risk of developing steroid-induced hyperglycemia. However, even short courses of steroids can lead to hyperglycemia.4 Patients with pre-existing diabetes or glucose intolerance are particularly susceptible to developing steroid-induced hyperglycemia.5
The clinical presentation can vary from asymptomatic to severe hyperglycemia with ketosis or hyperosmolar hyperglycemic state (HHS). HHS is a life-threatening complication of steroid-induced hyperglycemia that requires prompt medical attention.6 The management of steroid-induced hyperglycemia involves close monitoring of blood glucose levels and initiating appropriate treatment strategies.
Monitoring blood glucose levels in patients receiving steroids, particularly those with risk factors for diabetes or glucose intolerance, is crucial in detecting and managing steroid-induced hyperglycemia. Regular monitoring helps in early identification of hyperglycemia and allows for timely intervention to prevent complications. The American Diabetes Association (ADA) recommends frequent blood glucose monitoring, particularly in patients receiving high-dose steroids or those with pre-existing diabetes or glucose intolerance.7
Once diagnosed, the treatment approach may vary depending on the severity of hyperglycemia. In mild cases, dietary modifications and oral hypoglycemic agents can be employed to control blood glucose levels. In moderate-to-severe cases of steroid-induced hyperglycemia, blood glucose levels are significantly elevated and may not be adequately controlled with dietary modifications and oral hypoglycemic agents alone. Such cases often require more intensive management to achieve glycemic control and prevent complications. According to the ADA Standards of Care (2024), insulin therapy should be considered for patients when glycated hemoglobin (HbA1c) levels exceed 9–10%, or if oral medications are insufficient in controlling blood glucose levels effectively. Insulin becomes necessary in these situations to more precisely manage blood glucose levels and mitigate the risk of complications associated with uncontrolled hyperglycemia.8 Insulin therapy maintains blood glucose levels within a target range to minimize the risk of hypoglycemia. Insulin therapy can be initiated using subcutaneous insulin injections or continuous intravenous insulin infusion. The choice of insulin regimen depends on the individual patient’s needs and the clinical setting. In recent years, long-acting insulin analogs, such as insulin glargine, have gained popularity for managing steroid-induced hyperglycemia due to their favorable pharmacokinetic profile.9
The goals of this case report are to illustrate the clinical course, management strategies, and outcomes of steroid-induced hyperglycemia in a patient with rheumatoid arthritis. By presenting this case, the authors aim to highlight the challenges in managing hyperglycemia induced by steroid therapy, even for short durations. Understanding the nuances of such cases is crucial for healthcare providers to develop effective treatment plans tailored to individual patient needs. The importance of these cases lies in their ability to underscore the necessity for vigilant monitoring and individualized management of steroid-induced hyperglycemia. As steroid use is common in treating various conditions, recognizing and addressing hyperglycemia early can significantly impact patient outcomes. Collaborative efforts among rheumatology, endocrinology teams, and clinical pharmacists are essential in ensuring optimal disease control while minimizing long-term steroid use. These cases provide valuable insights into best practices for managing steroid-induced hyperglycemia and underscore the importance of patient education and interdisciplinary collaboration in achieving favorable outcomes.
CASE REPORT
A 58-year-old female patient with a history of rheumatoid arthritis presented to the Rheumatology Department with complaints of worsening joint pain and swelling. She had been previously diagnosed with rheumatoid arthritis 6 years ago and had been managed with disease-modifying antirheumatic drugs (DMARD) and occasional courses of oral steroids during disease flares. Due to worsening symptoms and evidence of disease activity on clinical examination and laboratory tests, the decision was made to initiate a short-term course of oral prednisone. The patient was started on oral prednisone at a dose of 20 mg/day for two weeks, followed by a tapering dose over the next four weeks. She was advised to continue her regular DMARD therapy during this period. The patient had no history of hypertension, diabetes, or coronary artery disease.
Clinical Course
Within a week of starting prednisone, the patient reported increased thirst, frequent urination, and fatigue. A fingerstick blood glucose test, performed at home, revealed a reading of 220 mg/dL. The patient contacted the department and was advised to come for further evaluation. On examination, the patient appeared fatigued but otherwise stable. Her vital signs were within normal limits. Laboratory investigations revealed a fasting blood glucose level of 240 mg/dL and a glycated HbA1c level of 8.5%. Additional laboratory tests, including renal and liver function tests, were within normal limits. Based on the clinical presentation and laboratory findings, the patient was diagnosed with steroid-induced hyperglycemia. The decision was made to monitor blood glucose levels closely and initiate dietary modifications with medication adjustments to achieve glycemic control.
Management and Follow-Up
Upon diagnosis of steroid-induced hyperglycemia, the patient was initially managed with dietary modifications and oral hypoglycemic agents. Specifically, she was prescribed metformin 500 mg twice daily and glipizide 5 mg once daily (Table 1). Despite these interventions, her blood glucose levels remained elevated. Given the persistence of hyperglycemia, insulin therapy was initiated when the patient’s HbA1c level reached 8.5%. A basal-bolus insulin regimen was chosen, starting with insulin glargine at a dose of 10 units daily for basal coverage and insulin lispro at a dose of 4 units before each meal for prandial control. These doses were adjusted based on regular blood glucose monitoring. Throughout the follow-up period, the patient’s blood glucose levels were closely monitored, and her insulin doses were titrated accordingly. Collaborative efforts between the rheumatologist, endocrinologist, and clinical pharmacists were crucial in optimizing her disease control while minimizing the long-term need for steroids. Regular follow-up appointments ensured that the patient’s glycemic control was maintained, and her symptoms improved significantly. The patient was advised to follow a balanced diet with a reduced intake of simple carbohydrates and sugary beverages. She was educated about the importance of portion control and regular meal timings. Additionally, she was referred to a registered dietitian for detailed dietary counseling and meal planning. Considering the moderate severity of hyperglycemia and the need for prompt glycemic control, insulin therapy was initiated. The patient was started on a basal-bolus insulin regimen using subcutaneous insulin injections. Basal insulin (glargine) was administered once daily at bedtime, while rapid-acting insulin (lispro) was administered before meals based on a carbohydrate-counting approach. The initial insulin doses were titrated based on the patient’s blood glucose levels and self-monitoring.
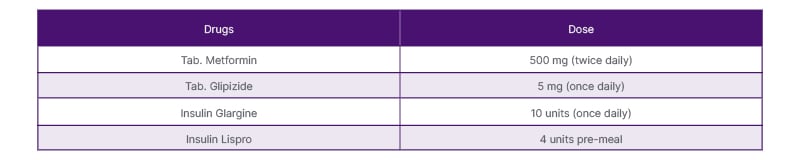
Table 1: Medications.
Regular follow-up appointments were scheduled to monitor the patient’s glycemic control and adjust insulin doses as needed. During these visits, blood glucose levels were closely monitored, and adjustments were made to the insulin regimen to achieve optimal glycemic control while minimizing the risk of hypoglycemia. Over several weeks, with close monitoring and appropriate insulin titration, the patient’s blood glucose levels gradually improved. Her fasting blood glucose levels stabilized around 100–130 mg/dL, and her postprandial blood glucose levels remained within the target range of 140–180 mg/dL (Table 2). The patient reported improvement in her hyperglycemia symptoms and felt more energetic. During follow-up visits, the patient’s overall health status, including her rheumatoid arthritis, was assessed. Adjustments were made to her DMARD therapy to optimize disease control while minimizing the need for long-term steroid use.
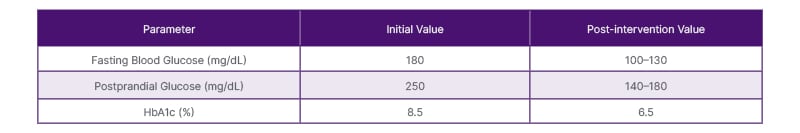
Table 2: Laboratory Findings.
DISCUSSION
Steroid-induced hyperglycemia is a well-recognized side effect of steroid therapy. It occurs due to the complex interplay of multiple mechanisms, including insulin resistance, impaired glucose uptake in peripheral tissues, and increased hepatic glucose production.10-12 The prevalence of steroid-induced hyperglycemia varies depending on factors such as the dose and duration of steroid therapy, as well as individual patient characteristics.12-13
Steroids, like prednisone, possess potent anti-inflammatory properties and are commonly used to treat various medical conditions, including rheumatoid arthritis, asthma, and inflammatory bowel disease.10-11 However, the use of steroids is associated with a wide range of adverse effects, including hyperglycemia. The risk of developing steroid-induced hyperglycemia is influenced by several factors, such as the dose and duration of steroid therapy, underlying patient characteristics, and concurrent use of other medications.14-16 The mechanisms underlying steroid-induced hyperglycemia are multifactorial. Steroids can impair insulin sensitivity and glucose uptake in peripheral tissues, leading to insulin resistance. They can also stimulate hepatic gluconeogenesis, which increases hepatic glucose production.11-12 These mechanisms contribute to elevated blood glucose levels in patients receiving steroids, ultimately leading to steroid-induced hyperglycemia. Dietary modifications play a significant role in the management of steroid-induced hyperglycemia. Patients are advised to follow a balanced diet with reduced intake of simple carbohydrates and sugary beverages. Portion control and regular meal timings are emphasized to help stabilize blood glucose levels.
Insulin therapy is often required in moderate-to-severe cases of steroid-induced hyperglycemia to achieve glycemic control. The choice of insulin regimen depends on individual patient characteristics and the clinical setting. Subcutaneous insulin injections and continuous intravenous insulin infusion are the two main approaches to insulin therapy. In recent years, long-acting insulin analogs, such as insulin glargine, have gained popularity for managing steroid-induced hyperglycemia due to their favorable pharmacokinetic profile.17-18 These analogs provide basal insulin coverage that mimics physiological insulin secretion and helps achieve stable glycemic control.
In this case, the patient developed hyperglycemia after starting a short-term course of prednisone for rheumatoid arthritis. This highlights the importance of recognizing and managing steroid-induced hyperglycemia in patients receiving steroid therapy, even for a limited duration. The decision to initiate a short-term course of prednisone was based on worsening symptoms and evidence of disease activity. However, the patient’s prediabetes history increased the risk of developing steroid-induced hyperglycemia. The patient had a history of rheumatoid arthritis and had been managed with DMARDs and occasional courses of oral steroids during disease flares. This background information further underscores the relevance of vigilantly monitoring blood glucose levels in patients with pre-existing conditions that increase the risk of hyperglycemia.
Subsequently, the patient developed symptoms of hyperglycemia, including increased thirst, frequent urination, and fatigue. The diagnosis of steroid-induced hyperglycemia was confirmed through blood glucose testing. Prompt recognition of these symptoms and timely diagnosis allowed for early intervention and management. The patient was educated about the importance of dietary modifications and was referred to a registered dietitian for detailed counseling and meal planning. Verbal informed consent was obtained from the patient for this case report. Lifestyle modifications, including diet adjustments, play a crucial role in managing steroid-induced hyperglycemia.
Considering the moderate severity of hyperglycemia, the decision was made to initiate insulin therapy in this case. A basal-bolus insulin regimen was chosen to provide basal insulin coverage and prandial insulin control, enabling a flexible and individualized approach to glycemic management. Close monitoring of blood glucose levels and regular adjustments of the insulin regimen based on the patient’s needs and monitoring results were implemented. Throughout the management process, the patient received extensive education about steroid-induced hyperglycemia. This included understanding the importance of blood glucose monitoring, the role of lifestyle modifications, and the use of insulin therapy. Collaborative efforts between the patient’s rheumatologist, endocrinologist, and clinical pharmacists were instrumental in optimizing disease control while minimizing the long-term need for steroid use.
CONCLUSION
In conclusion, this case underscores the need for healthcare professionals to be vigilant in monitoring and managing steroid-induced hyperglycemia in patients receiving steroids. Individualized treatment plans, interdisciplinary collaboration, and patient education are crucial in optimizing outcomes and minimizing the potential risks associated with steroid-induced hyperglycemia.







