Abstract
In recent years, a new concept of prehabilitation, enhancing an individual’s functional capacity ahead of a medical intervention, has begun to be explored in the fields of surgery and oncology, with positive results. This article explores applying the principle of prehabilitation to patients with rheumatoid arthritis prior to starting advanced therapies, including biologic disease-modifying antirheumatic drugs and targeted synthetic disease-modifying antirheumatic drugs. In this article, the literature is reviewed and the existing evidence is summarised, and the suggestion is that this approach could improve a patient’s chance of achieving low disease activity or remission.
There are a number of opportunities for improving the likelihood of patients with rheumatoid arthritis having a good response to therapy. Research shows that smokers starting TNF inhibitors are less likely to achieve a good response compared to non-smokers. Obese patients are also less likely to achieve a good response with TNF inhibitors; female patients with obesity may be less likely to achieve a good response with tocilizumab and early real-world data suggest there may be a reduced response to JAK inhibitors. Rheumatoid arthritis patients experiencing depression are less likely to respond to TNF inhibitors. Increased physical activity is potentially beneficial for all rheumatoid arthritis patients, although the effect on response to specific drugs has been less widely explored.
Prehabilitation approaches could include targeting smoking cessation, improving physical activity, providing psychological support, optimising BMI, and dietary changes. A number of studies have shown that each of these interventions can lead to significant improvements in disease activity scores, with some patients potentially benefitting from more than one intervention. The authors identify principles for delivering prehabilitation in practice and suggest that this is an exciting area for ongoing research.
INTRODUCTION
In recent years, there has been a growing body of evidence that prehabilitation, enhancing an individual’s functional capacity ahead of a medical intervention; encompassing medical optimisation, physical exercise, nutritional support, and stress/anxiety reduction has a positive impact on patient outcomes.1 So far, the focus has mainly been on the fields of surgery2 and oncology.3 In surgery, interventions to optimise functional capacity including modifiable risk factors such as anaemia, smoking, and anxiety, as well as exercise programmes to improve cardiovascular function and muscle function, have been shown to improve patient outcomes, with lower postoperative complication rates and earlier restoration of functional status.2 In oncology, prehabilitation interventions including exercise, nutrition, and addressing psychoeducational aspects of patient care have been shown to improve patient outcomes including cardiopulmonary function, lung function, and mood 30 days post-treatment. This seems to be particularly effective when combined with rehabilitation.3
Now is an opportune moment to examine how this concept could be applied in the field of rheumatology, with this article focussing on prehabilitation prior to the commencing of advanced therapies. Included are the biologic disease-modifying antirheumatic drugs (bDMARD) adalimumab, etanercept, certolizumab pegol, golimumab, abatacept, tocilizumab, and rituximab; a targeted synthetic disease-modifying antirheumatic drug (tsDMARD); and the JAK inhibitors (JAKi) tofacitinib, baricitinib, upadacitinib, and filgotinib for rheumatoid arthritis (RA). Adalimumab, etanercept, certolizumab pegol, and tocilizumab are licensed for the treatment of moderate-to-severe RA. Infliximab, golimumab, abatacept, and rituximab are licensed for use in combination with methotrexate for the treatment of moderate-to-severe active RA. Tofacitinib, baricitinib, and upadacitinib are licensed for the treatment of moderate-to-severe RA. The European Medicines Agency (EMA) is currently evaluating filgotinib as a treatment for active RA.
An estimated 60-70% of patients with RA respond to bDMARD,4 and the newer tsDMARD show similar response rates.5,6 This leaves 30-40% of patients who do not respond adequately, meaning their inflammatory arthritis remains uncontrolled and requires them to switch to other therapies. One approach to deal with this involves the use of stratified medicine approaches based on the accurate phenotyping of patients with RA using genomic, proteomic, and synovial membrane histological phenotyping. The authors suggest that another way to address the high level of non-responders to treatment involves assessing all modifiable factors that could be improved in advance of therapeutic interventions to maximise the likelihood of success. This article reviews the literature and provides a practical guide to how attending healthcare professionals can optimise patients’ chances of responding to advanced therapies using the concept of prehabilliation. This will not only provide patients with the best chance of achieving disease remission, but by using prehabilliation, individuals can be empowered to modify their own behaviours to help treat their disease. Approaches used to support patients’ prehabilitation are summarised in Figure 1.
Figure 1: Prehabilitation approaches to support patient.
SMOKING
Smoking is associated with an increased risk of structural damage progression in RA, with a cross-sectional study showing that smokers were significantly more likely to have joint space narrowing and erosions (p<0.05).7 Because of this, smoking status should be assessed in all patients and reassessed at regular intervals. Smoking is the most modifiable and predictive factor for TNF inhibitor (TNFi) treatment currently known. Registry data have shown an odds ratio of 0.52 (95% confidence interval [CI]: 0.29-0.96) for good response compared to non-smokers at 3 months,8 and a prospective study showed greater improvement in disease activity score 28 (DAS28) for non-smokers compared to smokers at 3 months (0.28 greater improvement; 2.64 versus 2.07; p=0.002).9 Past smoking did not affect the response rate, suggesting that if patients can be supported in stopping smoking their likelihood of a good response can revert to that of a non-smoker.8 Interventions targeting smoking prevention therefore have the potential to improve TNFi response rate, as well as vastly improving the patient’s overall health status.
Smoking status with other bDMARD has been less well studied, but an observational study did not show a difference in response to tocilizumab,10 and a large multi-national study looking at smoking status and response to rituximab also did not support smoking status as a predictor of response.11 The effect of abatacept is unknown. JAKi therapies have less available data, but one study has shown that current smokers treated with baricitinib have significantly increased radiographic progression, using the modified total Sharpe score, at 52 weeks (p=0.037).12 Although, a study pooling data from five Phase III trials including 3,315 patients did not find any significant difference in response to tofacitinib, regardless of baseline smoking status.13
All smokers should be offered support to quit smoking prior to commencing advanced therapies, with recent National Institute for Health and Care Excellence (NICE) guidelines including a range of evidence-based interventions, such as behavioural therapy; nicotine replacement; bupropion; a noradrenaline-dopamine reuptake inhibitor, which has been shown to be an effective add-on treatment in smoking cessation; and varenicline, a partial nicotinic acetylcholine receptor agonist.14
PHYSICAL INACTIVITY AND POOR FITNESS
Physical exercise has multiple beneficial effects for patients with RA, including improved cardiovascular fitness, improved cognitive function, increased bone density, and reduced fatigue,15 and all patients should be encouraged to include aerobic and resistance exercise as part of their routine care. Several exercise programmes have also been shown to improve disease activity scores in RA, with no evidence to suggest that increased activity will trigger a disease flare.15,16 One study found that a short-term intensive exercise programme, involving high-intensity interval walk training, for patients with active RA was more effective in improving muscle strength than a conservative exercise programme.16 Another study found a reduction in DAS28 of 0.7 in patients undergoing a 10-week intensive programme compared to the control group who received usual care (DAS28 2.4 versus 3.1; p=0.001).17 European League Against Rheumatism (EULAR) guidelines recommend that physical activity promotion should be an integral part of the management of patients with inflammatory arthritis and state that healthcare professionals have a responsibility to promote and facilitate physical activity for patients.18 Sarcopenia is more prevalent amongst RA patients, with approximately 40% of RA patients being defined as sarcopenic (a relative skeletal mass index of <5.5 kg/m2 for females and <7.26 kg/m2 for males). Sarcopenic RA patients are more likely to have increased cardiovascular events, as well as progressive erosive changes on X-ray, suggesting that interventions to improve muscle mass could be of benefit.19 The effect of exercise interventions prior to the initiation of RA treatments and the impact on efficacy has not been widely studied. Although there is evidence that physical exercise improves muscle tone, bone mass, and cognitive function, as well as aiding weight loss, there is currently no evidence that physical activity has a direct effect on an RA patient’s response to bDMARD.
NUTRITION AND BMI
An increasing number of patients are overweight (BMI >25), and recent World Health Organization (WHO) data have classified 58.3% of European adult males and 51.2% of European adult females as overweight, with the number predicted to continue rising.20 Obesity is considered as a mild chronic inflammatory state; adipose tissue produces cytokines such as TNF and IL-6 having proinflammatory properties.21
Obese patients with RA have a significantly reduced chance of remission with TNFi treatment, with a meta-analysis showing that the odds of failing TNFi therapy being up to 60% higher in patients with a high BMI,22 and registry data showing improvements in DAS 28 being 0.22 lower in obese patients receiving TNFi at 6 months (-0.22; 95% CI: -0.42 to -0.03).23 Obesity did not impact on the efficacy of abatacept or rituximab (cell-targeted therapies).23,24 For tocilizumab, some studies have shown reduced efficacy in overweight female patients;23 however, recent data from the American Corrona® RA registry suggest that BMI has no effect on the response to treatment with tocilizumab.25 Some early real-world data suggest that obesity predicts a poor response to the JAKi baricitinib and tofacitinib, with low disease activity reached by 42% of patients with normal weight, but only by 19% of obese patients, at 6 months.26 Figure 2 depicts a suggested treatment algorithm for obese patients going onto advanced therapies.
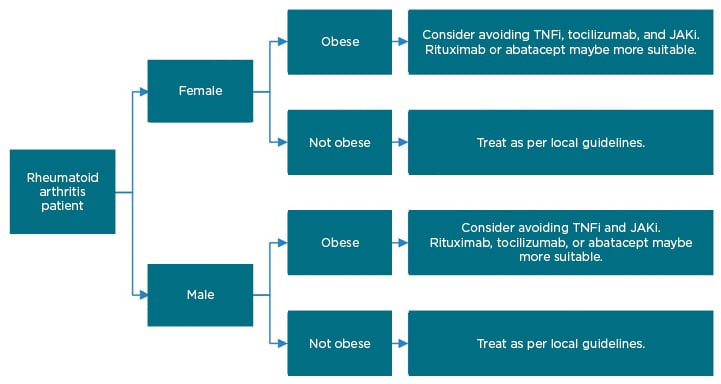
Figure 2: Suggested treatment algorithm for patients going onto advanced therapies for rheumatoid arthritis.
JAKi: JAK inhibitor; TNFi: TNF inhibitor.
Addressing weight management is challenging. To improve the effectiveness of advanced therapies and reduce the overall disease burden, particularly in patients receiving TNFi medications, consideration needs to be given to achieving an optimum and healthy BMI, ideally before starting the advanced therapy. This can be achieved by having healthy open discussions between the clinicians and patients, including an exploration of their perceptions and motivations. Consideration can also be given to providing support, for example referring patients to dieticians, physiotherapists, support groups, and other services available in the community to help reduce weight.
RA patients are at significantly increased risk for osteoporosis and bone loss because of RA disease processes and glucocorticoid use and RA patients are at increased risk of fragility fractures.27,28 RA has therefore been incorporated as a dichotomous predictor in the WHO fracture risk assessment (FRAX) algorithm for predicting the 10-year risk of hip or major osteoporotic fracture.29 RA patients should have their bone health assessed at regular intervals, including vitamin D levels, with appropriate calcium and vitamin D supplementation being prescribed if indicated. The FRAX tool can be used to evaluate 10-year risk of fragility fracture and aid decision-making regarding additional treatments, such as bisphosphonates.
The role of diet and the gut microbiome and the initiation of RA in genetically susceptible patients is of ongoing interest.30 One study showed that RA patients with higher plasma n-3 polyunsaturated fatty acids levels when starting etanercept had lower DAS 28 scores at 3 months (-0.51; p=0.007). In vitro etanercept treatment led to an increase in IL-17 expression, but this increase was not seen in patients with high plasma n-3 polyunsaturated fatty acids levels, suggesting a possible mechanism of action.31
A meta-analysis has not shown any conclusive evidence for the use of probiotics in RA.32 Likewise, no conclusive evidence has been found for a therapeutic effect of dietary fibres and wholegrains, fruit, spices, or of elimination of gluten or meat.33 Further research is being carried out in this area to look at the effect of dietary supplementation.
Excess sodium is known to increase the differentiation and activation of Th17 cell pathways by inducing serum glucocorticoid kinase 1.34 Marouen et al.35 sought to explore this by comparing sodium intake in RA patients with matched controls. Sodium intake was evaluated by 24-hour urinary sodium excretion. Sodium excretion was greater for patients with early RA than controls, even once confounding factors were accounted for (p=0.043). Additionally, patients with radiographic erosion at the time of diagnosis had a higher sodium excretion than those without (p=0.028). Further interventional studies looking at the effect of sodium restriction in the diet on RA disease activity and progression would be of benefit.
PSYCHOLOGICAL SUPPORT
Depression commonly occurs with RA, affecting 13-20% of patients, which is 2- to 3-times higher than the prevalence in the general population.36 Both health assessment questionnaire (HAQ) score and pain score reporting are strongly influenced by depression, with depressed patients scoring 0.25 HAQ units higher than non-depressed patients and depressed pain giving a visual analogue pain score of 1.9 higher, when scoring from 0 to 10 (p<0.0001).37,38 Persistent depression is poorly recognised in rheumatology clinics and is associated with a poorer response to TNFi, with one study showing a 0.49 lower improvement in DAS28 at 3 months in patients with depression at onset of treatment compared to those without (DAS28 improvement 1.7 versus 2.2; p=0.005), with the difference observed because of both objective and subjective measures.39 Other studies have supported this finding,40,41 although a South Korean health insurance database did not show any difference in TNFi discontinuation rate between patients with depression and controls.42 There has been comparatively less research investigating non-TNFi bDMARD, but one study showed that RA patients with elevated plasma IL-6 and IL-17 levels were more likely to have symptoms of depression.43
Efforts should also be made to support patients in adhering to their medications. Several studies have shown that patients of all age groups, particularly the young, prefer subcutaneous administration of medication as compared to intravenous administration. Lack of localised skin reaction, reduced frequency of dosing, and fast onset of action are also important to patients.44 Patient preferences and beliefs have been shown to be important in adherence to medications, with patients considering whether their beliefs about the necessity of the medication outweigh any concerns regarding the potential adverse effects of taking them.45 Patients concerns and beliefs can be explored using several methods, including the Beliefs about Medicines Questionnaire (BMC),46 to help improve understanding, allay fears, and ultimately improve adherence. For patients to be able to use subcutaneous injections, they, or a relative or friend, will need to be able to have the manual dexterity to administer the drug and this may be challenging for some patients with chronic RA affecting their hand movements.
A Cochrane review of patient education in RA examined various interventions, including formal structured instruction on RA and ways to manage arthritis symptoms, psychobehavioural methods to promote changes in health behaviours, as well as instructional interventions including exercise, biofeedback, and psychosocial supports. The review showed a small but statistically significant improvement in scores for disability, pain, patient global status, psychological status, and depression.47 Other meta-analysis reviews support the idea that providing psychological support can be beneficial for patients, with one having shown that cognitive behavioural therapy can significantly reduce levels of anxiety (p=0.005) and depression (p<0.00001) and relieve fatigue symptoms (p=0.006) in RA patients;48 furthermore, another study showed that mindfulness interventions can reduce depression (p=0.02) as well as improve DAS28 scores (-0.29 improvement; 95% CI: -0.38 to -0.19); p<0.00001).49
DISCUSSION
bDMARD and tsDMARD have revolutionised the treatment of RA; however, there remains a significant number of patients who fail to achieve a good response to treatment. By targeting smoking cessation, physical activity, weight reduction, diet, and providing psychological support, we have the potential to improve the likelihood of patients responding to these drugs. Table 1 summarises different interventions and potential improvements in disease activity scores.
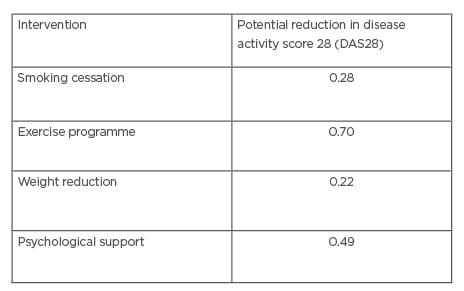
Table 1: Estimated improvements in disease activity score 28 (DAS28) score with prehabilitation intervention.9,17,23,39
There are a wide range of prehabilitation interventions available to help provide holistic care to patients prior to starting advanced therapies to help maximise a patient’s response. Many of these interventions are led by allied health professionals, and physicians would benefit from assessing the frameworks within their healthcare system to address how this multidisciplinary approach is implemented.50,51 There is good evidence that non-smokers achieve better response rates compared to smokers starting TNFi.8,9 Further evidence concerning the effect of smoking on JAKi would be of use and future studies and registry data may be able to guide this. Several evidence-based interventions exist, as outlined above, and starting an RA patient on an advanced therapy can provide an opportune moment to promote smoking cessation. Exercise programmes can provide a range of health improvements for RA patients, including cardiovascular capacity, muscle mass, bone density, and disease activity.15-17 Several exercise programmes have been studied in the literature and it may be of benefit for clinicians to work with allied health professionals locally, including physiotherapists, to help RA patients increase physical activity. Being obese predicts poor response to TNFi,22 being female and obese possibly predicts poorer response to tocilizumab,23 and response to JAKi may also be reduced for obese patients.26 Achieving a healthy BMI has a huge range of health benefits and aiding weight loss can be beneficial for an RA patient’s inflammatory arthritis as well as their overall general health. Engaging with patients’ general practitioners and local dietetics department may help provide practical assistance in achieving this. In terms of diet, RA patients have a significantly higher risk of osteoporosis and should have their bone health assessed at regular intervals and vitamin D and calcium supplementation should be considered. There is some interesting research looking at diet supplementation with polyunsaturated fatty acids and the effect on disease activity, and it will be of interest to see if further evidence supports their use in the future. Depression is more prevalent amongst RA patients and some studies have suggested that this is associated with a poorer response to TNFi, although this finding has not been replicated in registry data42 and further studies exploring this would be of use. Providing psychological support, in the forms of cognitive behavioural therapy and mindfulness, have the potential to reduce depression, anxiety, fatigue, and disease activity.48,49 This suggests that it would be of benefit for departments to identify patients who could gain benefit from psychological support and implement approaches to support them. If a patient is diagnosed with depression, it is also important to involve general practitioners and psychiatry services as appropriate. Finally, exploring patient preferences and beliefs is important in improving adherence to medications to help maximise response.
CONCLUSION
These prehabilitation interventions can improve our patients’ cardiovascular health, aerobic function, fatigue symptoms, and quality of life. These different prehabilitation approaches are likely to be interlinked and may in some cases be symbiotic, and it would be of interest to explore the cumulative effect of these approaches. There are many more questions to be answered regarding prehabilliation and it is an exciting area for further research. This article provides a guide to approaches that can be used right now in our clinics when prescribing advanced therapies to give patients the best chance of success.







