Abstract
Osteoarthritis (OA) is the most common form of arthritis, yet has historically lagged far behind rheumatoid arthritis in terms of drug development. Despite the many challenges presented by clinical trials in OA, improvements in our understanding of disease pathogenesis and a move to treat pain, as well as underlying disease process, mean there are now many new pharmacological therapies currently in various stages of clinical trials. The medical need for these therapies and the evidence for recent tissue and molecular targets are reviewed. Current therapeutic examples in each area are discussed, including both novel therapeutics and existing agents which may be repurposed from other disease areas. Some challenges remain, but opportunities for improving symptoms and disease process in OA in the clinic with new pharmacological agents would appear to be on the close horizon.
INTRODUCTION
Osteoarthritis (OA) is the most common form of arthritis, causing enormous suffering and healthcare cost; one-third of those aged >45 years seek treatment for OA and 81% of these have constant pain or limitation of activities,1 with 7.5 million working days lost per annum in the UK alone. The majority of joint replacements can be attributed to OA pain. By 2030, it is expected that 560,000 hip replacements each year will occur within the USA.2 This being said, OA has historically lagged behind rheumatoid arthritis (RA) in levels of research and drug development. With an ageing and increasingly obese population, incidence of symptomatic OA and joint replacements are increasing year-on-year, associated with increasingly unsustainable costs.3 OA is now becoming a disease seen in younger people, in their 40s and 50s, who are not yet appropriate for joint arthroplasty. This has led to a clear and increasing unmet need for new pharmacological treatments. In previous decades, the desire to demonstrate structural modification has been hampered by several factors: the limited impact new agents have on the US Food and Drug Administration (FDA)-required endpoint joint space width on X-ray,4 toxicity issues in some promising drug classes such as MMP inhibitors,5 and the realisation that the placebo effect in OA (as in many diseases), is substantial and needs careful consideration in trial design.6 However, there is now great momentum internationally to develop better drug treatments for the condition, aided by significant advances in our understanding of disease pathogenesis. A number of agents across several drug classes seem set to transform the way we think about the medical treatment options of OA.
The Medical Need for New Pharmacological Agents for Osteoarthritis
Drug treatments for OA can be divided into those which improve pain or symptoms (SYMOADs) and those which improve structure, or slow progression, with or without an effect on pain (DMOADs). Existing guidelines for the management of OA include several pharmacological therapies;7-9 education, weight loss, and exercise advice should always precede and accompany any drug treatment of the disease. In those whose pain is not adequately controlled, first-line, evidence-based analgesia includes topical non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen (paracetamol). Oral NSAIDs and cyclooxygenase-II (COX-II) inhibitors, opiates, or intra-articular steroids can all be considered if these first-line agents fail. However, even the best of these agents only gives clinically meaningful efficacy in half of those taking the drug10 and the side effects and potential toxicities limit their use in a population who often have associated comorbidities. Several non-pharmacological treatments also exist, but are not the focus of this review. For those with advanced radiographic disease, substantial pain, and impaired quality of life, total knee or hip replacement gives substantial improvement in symptoms in the majority but carries the associated costs and risks of a large operation.3 Nonetheless, there still remains a significant treatment gap: those who are younger (<~55) with symptomatic disease and those of any age who have symptomatic disease which is not considered radiographically advanced enough for joint replacement (Figure 1). It is important to note that not all those with radiographic disease progress; new medical treatments might increase this group and reduce the numbers ultimately requiring surgery.
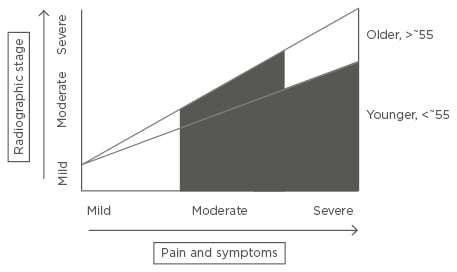
Figure 1: The medical treatment gap in osteoarthritis.
Existing, evidence-based treatments are often effective in those with early disease where there are mild symptoms, and also in older individuals with advanced radiographic disease with severe pain or other symptoms severely affecting quality of life (for whom joint replacement is a highly effective treatment). The medical need for novel pharmacological agents arises in those whose symptoms have not responded adequately to existing evidence-based interventions including existing pharmacological choices, or in whom these are contraindicated or not tolerated: younger patients with moderate-to-severe symptoms, with any radiographic stage of disease (for whom joint replacement surgery is not indicated); and also those older patients with moderate radiographic disease only, or those who do not wish for surgery. Marked in black, pharmacological interventions for the condition are likely to be aimed at the above treatment gap.
Scope of this Review
Progress has been reviewed from the last 5 years; only human, double-blind, randomised controlled trials (RCTs) published in peer-reviewed journals, or with published protocols or registration on clinical trials websites have been included. This is not intended to be an exhaustive, systematic review, but rather to encompass the key areas of interest with relevant examples. Many more targets are apparent from preclinical models that have not entered human studies; past experience tells us only a minority of these will prove translatable. All peripheral joints and stages of disease have been considered, however it is apparent that well-established/advanced knee OA is studied most commonly, with some studies in hip or hand OA. New formulations of drug classes in current clinical use, notably NSAIDs, steroids, or viscosupplementation, have not been included. Similarly, nutraceuticals and botanicals are not reviewed here, as they are subject to different regulation, and often have ill-defined and potentially multiple active ingredients which make them difficult to assess pharmacologically; an excellent review of this area has been carried out recently.11 Stem cell and cell-based therapies and platelet-rich plasma therapy are beyond the scope of this review.
TISSUE AND MOLECULAR TARGETS FOR OSTEOARTHRITIS
Overview
OA is now considered a disease of the whole joint, with most of the affected connective tissues being targets for novel therapeutics (Figure 2).12 Changes within articular cartilage occur early in the disease and include excessive action of proteinases (aggrecanases and collagenases) causing loss of proteoglycan and Type II collagen, both critical matrix molecules. Structurally compromised articular cartilage is then vulnerable to further damage.13,14 Bone remodelling occurs early in the disease, and closely mirrors changes within the articular cartilage.15 Bone marrow lesions (BMLs) evident on magnetic resonance imaging (MRI) are associated with pain and progression16,17 and new bone formation occurs, including osteophyte formation. This process has been somewhat underestimated on two-dimensional imaging and is often extensive and a hallmark of disease.18 Such remodelling appears to be a repair attempt; whether this is sometimes beneficial, stabilising or splinting a joint, or whether it is always a primary driver of pain and other symptoms, is a matter of debate.
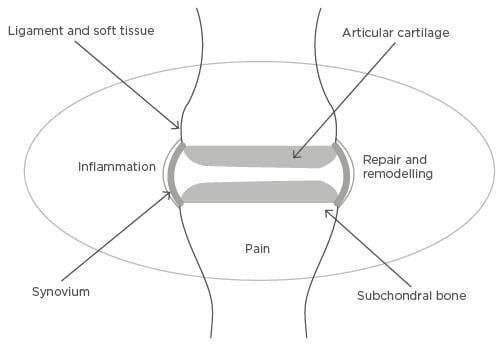
Figure 2: Tissue and molecular targets in osteoarthritis.
Osteoarthritis is a disease of the whole joint, with processes affecting articular cartilage, subchondral bone, synovium and ligament, and other soft tissue such as meniscus in the knee, all shown to be important in the pathogenesis of the disease. Three related molecular processes, inflammation, repair and remodelling, and pain-generating pathways, are important molecular targets in all of these joint connective tissues.
Therapeutic targets will be reviewed relevant to cartilage, bone, inflammation/repair, and pain, with examples of drugs targeting pain, structure, or both.
Cartilage
There has been long-standing emphasis on disease modification in articular cartilage. It was demonstrated in two reports in 2005 that the key enzyme responsible for the specific cleavage of aggrecan seen in mouse OA was a disintegrin and metalloproteinase with thrombospondin motifs-5 (ADAMTS-5); knockout of this gene significantly inhibited OA development.19,20 This also appears to be the important aggrecanase in humans.21,22 Several pharmaceutical companies have developed and tested antibodies or small molecule inhibitors to ADAMTS-5 or both ADAMTS-4/5 for their disease-modifying effects in OA.21,23 However, difficulties in demonstrating structural modification and potential toxicities have, to date, hampered successful outcome for any of these agents; aggrecanases are found at low levels in cardiovascular and nervous systems. However, some agents remain in clinical trials at present. Given that cartilage is aneural, it might be supposed that these agents may slow down progression but have no direct effect on pain. Promotion of pathways which regulate aggrecanase activity may prove more tractable in the future.
The Wnt signalling pathway is known to play a central role in joint tissue formation, including cartilage and bone, and altered Wnt signalling has been associated with cartilage loss. Some improvement in cartilage thickness and in knee pain has recently been reported in a small study of an intra-articular injection of Wnt inhibitor SM04690.24
Bone
The two drug classes lending strongest support to bone as a therapeutic target are bisphosphonates and strontium ranelate. Previous trials of risedronate did not meet primary endpoints on structure, but had significant effects on some secondary patient-reported outcomes and also had suppressive effects on C-terminal crosslinking telopeptide of collagen Type II (CTX-II), a qualified biomarker of cartilage turnover, which may also reflect changes in bone.4,25 More recently, two trials of systemically administered bisphosphonates reported significant effects on OA pain. Laslett et al.26 reported that individuals treated with intravenous zoledronate had reduced visual analogue scale (VAS) pain and BMLs over 1 year. Neridronate improved VAS pain and BMLs on whole organ MRI score.27 Intra-articular clodronate has also shown benefit.28 Bisphosphonate use was also associated with reduction in knee pain over 3 years in the Osteoarthritis Initiative (OAI) cohort.29
In an osteoporosis RCT, it was noted that there was substantial radiographic improvement of OA with strontium ranelate.30 In a subsequent RCT in knee OA (SEKOIA trial), effects of 1 g or 2 g of the drug were examined.31 Paradoxically, structure improved most at 1 g dosing, but pain benefit was only seen with 2 g. Bruyère et al.32 noted a clinically meaningful improvement in pain, function, and stiffness at the 2 g dose. In post-hoc analyses using MRI outcomes, both doses had significant effects on BMLs.33 Similarly, if patients were stratified post-hoc by meniscal extrusion, there was quantitative MRI evidence of structural disease slowing.34 In 2014, the drug was restricted for its primary indication of osteoporosis, due to safety concerns relating to risk of venous thromboembolism and myocardial infarction. As a result the programme in OA is currently on hold.
There have also been negative studies in this area. Salmon calcitonin, another osteoporotic agent, did not show significant difference from placebo in a trial of knee OA.35 There was much circumstantial evidence that vitamin D would reduce pain and potentially disease-modify. However, in two recent studies, oral vitamin D raised serum levels significantly, but had no significant difference on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) classified pain or cartilage volume.36,37
Inflammation
Inflammation in OA remains a surprisingly contentious topic. There is evidence that inflammation is important in initiation of disease, in early disease, and in late disease. Mechanical activation of inflammatory signalling pathways and inflammatory response genes in joint connective tissues appears necessary to drive a process leading to OA in preclinical models.38,39 In longitudinal studies, elevated systemic levels of inflammatory cytokines (such as interleukin [IL]-6 and tumour necrosis factor [TNF]-α) are associated with disease progression.40,41 These molecules are capable of inducing activation of aggrecanases. A number of connective tissues including cartilage are capable of their synthesis.42,43 In established disease, synovial and fat pad inflammation would appear to be one of the strongest predictors of both pain and progression.44,45 Existing drugs with proven efficacy on pain by anti-inflammatory action (NSAIDs, steroids) are not thought to be disease-modifying.
Several anti-cytokines licensed for use in RA have also been studied in OA. Perhaps the only surprise is that there is still an incomplete picture. In a small RCT of the anti-TNF adalimumab in hand OA, 50% pain reduction was seen in 35.1% of the active arm and 27.3% of the placebo arm, with the conclusion that adalimumab was not superior to placebo.46 In a previous study Verbruggen et al.47 had reported a subgroup of hand OA with palpable soft tissue swelling (at highest risk of erosion) had statistically less radiological progression in the first 6 months if treated with adalimumab. Subcutaneous etanercept was not significantly better than placebo in a RCT in erosive hand OA on the primary outcome of hand pain. However, when analysed per protocol, the etanercept group with symptomatic disease who completed the study had significantly improved pain and less structural damage.48 More recently, a small study of a single injection of intra-articular etanercept for knee OA showed significant reduction in pain VAS compared with intra-articular hyaluronan.49 To date in knee OA, there has only been an open label study of a systemically administered anti-TNF.50 A human monoclonal antibody to IL-1R1 was reported as showing no discernible benefit versus placebo in 159 individuals with knee OA over 12 weeks.51 A Phase IIa RCT of the safety and efficacy of ABT-981 (neutralising antibody to IL-1α and IL-1β) in hand OA is ongoing.52 An IL-6 receptor antagonist is also being investigated in hand OA in a French study.53 The effects of this drug class on large joint OA are unreported. A selective oral inducible nitric oxide synthase (iNOS) inhibitor, cindunistat, showed no slowing of joint space narrowing and no impact on pain or function.54
Such cytokines may implicate innate immunity including macrophages in OA development. New Phase II trials of an antagonist of granulocyte macrophage-colony stimulating factor (GM-CSF) are being carried out in hand OA, in addition to RA.55 The NALP3 inflammasome, which is implicated in gout, appears to be activated in OA by crystals or activation by danger-associated molecular patterns.56-58 Colchicine is an old drug which has its effect partly via the inflammasome. The efficacy of this drug on symptomatic post-menopausal knee OA is being assessed.59 Impaired microvasculature may be important in predisposing individuals to OA.60 It is possible that other drugs which have a vascular anti-inflammatory effect may benefit OA: atorvastatin, low molecular weight heparin,61 the aldosterone antagonist spironolactone,62 and the sodium channel blocker VX-15063 have been, or are being, tested for their effects on OA.
Inhibition of synovial inflammation is likely to be an important mode of action for some of these agents. Repurposing studies have been carried out of existing anti-synovial agents to assess their effect on painful OA. In an Egyptian RCT in a pre-defined group with knee OA and inflammatory signs such as effusion, 25 mg methotrexate was superior to placebo on knee pain and function; it also reduced ultrasound-evident inflammation.64 A further UK-based trial assessing this drug in a wider knee OA population non-responsive to existing therapies, is in progress.65 Hydroxychloroquine was not found to be acceptable or show pain improvement in a small study which was terminated early.66 Two large European trials have subsequently assessed this drug in somewhat different populations with hand OA; in a multicentre RCT, hydroxychloroquine was not significantly different to placebo on the primary outcome of average hand pain and no differences could be seen in a subgroup with inflammatory change on ultrasound at baseline.67-69
Repair
Pro-repair and anti-catabolic pathways are activated as part of the inflammatory response; harnessing the effects of these pathways may be therapeutically important. Two well-described reparative or anti-catabolic pathways activated in OA and injured connective tissues, are fibroblast growth factor (FGF) and transforming growth factor beta (TGF-β). There is much to learn about the relative roles of multiple family members and their receptors. Both pathways are pleiotropic: knocking out FGF-2 accelerates murine OA, but FGFs contribute to the inflammatory signalling response to connective tissue injury.43,70 Similarly, TFG-β and its family may be anti-catabolic for articular cartilage and promote cartilage growth, but also promote bone growth (promoting osteophytes) and fibrosis.71 The first published clinical trial in this area tested intra-articular FGF-18 (sprifermin) in knee OA; the agent was well-tolerated. The primary quantitative MRI endpoint was not met; however, all active treatment groups had improved pain scores, with significant difference from placebo at 12 months.72 Post-hoc analysis showed reduced cartilage loss and increased cartilage thickness in the active arm.73 As such, the drug will proceed to Phase III assessment. Antibodies to TGF-β exist and are also of therapeutic interest, but arguably only if they can be targeted to act in a tissue-specific manner.71
Pain
Pain as a primary outcome in clinical trials is now more accepted and tractable than structural modification in established disease, and has led to a recent increase of RCTs within this area. Pain is the leading symptom for patients; whilst it may be driven by changes in specific joint tissues such as bone and synovium, there is evidence that targeting primary neurotrophic pathways activating sensory afferents may be a highly effective way of relieving pain in this and other chronic painful conditions. There is evidence from mouse models that nerve growth factor (NGF) and other neurotrophins are over-expressed in symptomatic disease, and are synthesised by the joint connective tissues themselves.74 NGF causes allodynia and hyperalgesia is often associated with the disease. Inhibition of NGF or calcitonin gene related peptide (CGRP) reduces pain behaviour in preclinical models.75,76
In humans, the most studied area is NGF inhibition.77 In a seminal RCT in 2010, Lane et al.78 showed that subcutaneous administration of tanezumab, a monoclonal antibody to NGF, brought about significant, dose-related, clinically substantial relief of pain on walking in knee OA when compared with placebo. Subsequent trials have demonstrated superiority to NSAIDs and opiates.79,80 Other monoclonal antibodies to NGF such as fasinumab and soluble tropomyosin receptor kinase A (TrkA) receptor fusion proteins have demonstrated similar effects.81-83 An FDA halt to Phase III trials due to a potential safety signal of osteonecrosis has now been lifted. Two out of eighty-seven adjudicated cases were likely due to osteonecrosis. Most were in fact cases of rapidly progressive OA (RPOA), which was primarily associated with concurrent chronic NSAID administration.84 There was also increased risk on the highest dose (10 mg of tanezumab), or where there was identifiable risk for RPOA (subchondral insufficiency fractures, very advanced radiographic disease). There was no apparent association with greater pain relief or anaesthesia in these individuals. New Phase III trials mitigating against RPOA risk, with increased neurological monitoring have now recommenced.77,84 Small molecule inhibitors selectively inhibiting the NGF receptor, TrkA that are orally available and shorter-acting are also being tested; safety considerations will need to be similar for all of these agents.
Despite promise from preclinical models, a monoclonal antibody to CGRP (LY2951742) showed no dose response, and no superiority to celecoxib.85 An inotropic glutamate receptor antagonist failed to meet its primary endpoint. This was a short study with an active comparator, pregabalin.86 The selective serotonin re-uptake inhibitor (SSRI) duloxetine has been more extensively studied. Two studies showed significantly improved pain compared with placebo, but no benefit of 120 mg over 60 mg.87,88 Further studies are comparing the effects of pregabalin and duloxetine in hand OA89 and their mechanism of action.90
LIMITATIONS AND PERSPECTIVES
We continue to learn lessons from recent clinical trials in OA: firstly, that we must continue to understand the basic mechanisms in the disease to predict drug effects. Structure-modifying agents could potentially be combined with a pain-relieving agent to improve patient acceptability, but the anti-NGF trials tell us we must first fully appreciate potential interactions. Secondly, there is always a balance to find in acceptability, safety, and effectiveness. What is acceptable risk in conditions such as OA? Some patients may accept a very low risk of acceleration of their disease towards joint replacement or other potential toxicity, if a drug provides markedly improved quality of life. A high level of patient involvement in assessing acceptability and approval of new drugs for OA is needed, bench-marking on existing drug classes such as NSAIDs and opiates. Repurposing of existing agents (with acceptable safety profiles for other indications and potentially relevant effects in OA) is likely to be an expanding and cost-effective area.
Design of OA trials has in recent years been a bigger challenge than the identification of novel targets.91 Better trial design needs to take account of and minimise placebo response; we need better, more reliable patient and disease-relevant outcome measures. One of the biggest challenges in this area is the heterogeneity of the disease, in its phenotype and progression. We need to be able to stratify patients in our recruitment to trials, enriching for those at highest risk of progression (of symptoms or structure) or with relevant disease phenotype.92 Better prognostic modelling is being developed.12,93 These groups may include those with early disease, or those at risk of disease, such as those with knee trauma, where ~50% of individuals will develop symptomatic radiographic disease.94 The gain may arguably be larger in these groups, in advance of extensive irreversible structural damage. Most trials to date have been in the knee and hip. The noticeable increase in trials of agents in hand OA will diversify the field; we may discover that some agents have site-specific effects. It may be necessary to revisit some failed trials once we have better ways of assessing effects of some of these agents.
Who will benefit from these drugs? The majority of patients with OA may not need these drugs. Rather, they will be reserved for those with highly symptomatic and/or progressive disease who have failed to respond to primary interventions.7 We will need to personalise certain treatments to particular groups, by better defining disease phenotypes, with valid biomarkers for disease processes or response to a particular drug class.95 More expensive biological interventions will only find their place if they prove to be cost-effective, for example if they delay or reduce the need for joint replacement or other comorbidity or mortality risk associated with OA.96 Specialist drugs will likely require eligibility assessment and monitoring, much like DMARDs and biologics for RA: we need to develop a readiness to think about the care of this disease in a different way.
CONCLUSIONS
Opportunities for modifying symptoms and disease process in OA with new pharmacological agents would appear to be on the close horizon. There are a variety of tissue and molecular targets, old and new. A renewed focus on patient-reported symptoms, particularly pain, has brought about the possibility of new drug classes for OA. The question would appear not to be whether, but simply when; a new approved class would seem likely within 5–10 years. Understanding what impact new agents have on the underlying disease process, and what they can teach us about disease pathogenesis is an essential part of their assessment.








