INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in general leads to a mild disease in children, but a rare yet serious complication of multisystem inflammatory syndrome in children (MIS-C) has been reported.1 MIS-C cases are on the rise during the COVID-19 pandemic, and clinical features overlap between MIS-C and an already well-known clinical entity in children: Kawasaki disease (KD). Both KD and MIS-C are diagnoses of exclusion, and can present with acute fever and increased inflammatory markers without any other potential aetiology.2 Despite the overlap in clinical presentation, there are some differentiating features that could help establish an accurate diagnosis and appropriate management, which are highlighted in this article.
CASE DEFINITIONS
The Centers for Disease Control and Prevention (CDC) case definition of MIS-C is an individual aged <21 years presenting with fever of >38.0 °C lasting >24 hours, laboratory evidence of inflammation, clinically severe illness requiring hospitalisation, with multisystem (>2) organ involvement; no alternative plausible diagnoses; and positive result for current or recent SARS-CoV-2 infection by reverse transcriptase-PCR, serology, or antigen test; or exposure to a suspected or confirmed COVID-19 case within the 4 weeks prior to the onset of symptoms. Laboratory evidence of inflammation includes, but is not limited to, one or more of the following: an elevated C-reactive protein, erythrocyte sedimentation rate, fibrinogen, procalcitonin, D-dimer, ferritin, lactic acid dehydrogenase, or IL-6; neutrophilia; lymphopenia; and hypoalbuminaemia.3 The World Health Organization (WHO) case definition of MIS-C differs from that of CDC by age criteria of 0–19 years and fever >3 days.4
KD is a vasculitis of medium-sized blood vessels that presents as an acute illness in a patient with a fever of ≥5 days duration and the presence of ≥four of the five principal clinical features that include: erythema and cracking of lips, strawberry tongue, and/or erythema of oral and pharyngeal mucosa; bilateral bulbar conjunctival injection with no exudate; maculopapular, diffuse erythroderma, or erythema multiforme-like rash; erythema and oedema of the hands and feet in the acute phase, and/or periungual desquamation in the subacute phase; and cervical lymphadenopathy (≥1.5 cm diameter). Patients whose illness does not meet the CDC case definition but who have fever and coronary artery abnormalities are classified as having atypical or incomplete KD.5
CLINICAL FEATURES
KD often affects young children <5 years and is more common in the Asian population.5 However, for MIS-C, the average age of presentation is 9–11 years, and it is more common in children from Black and Hispanic backgrounds, which could be because of increased incidence of SARS-CoV-2 infection in these subgroups.6,7 Seasonal variations are noted in KD, and it is more common in winter and spring. Temporal association between MIS-C cases and an increase in the number of SARS-CoV-2 cases has been noted since the beginning of the pandemic; MIS-C usually presents 4–5 weeks after SARS-CoV-2 infection, which is itself usually mild.2,6-8 Differentiation can be difficult based on clinical features alone, as both KD and MIS-C can present with fever, increased inflammatory markers, and mucocutaneous manifestations. However, in MIS-C, cardiac markers such as B-type natriuretic peptide and troponin are increased, with associated thrombopenia, leukopenia, and lymphopenia; whereas, thrombocytosis is common in KD. Coagulopathy, gastrointestinal symptoms, neurocognitive symptoms, and diastolic dysfunction with cardiogenic shock are more common in patients with MIS-C compared to KD.1,2
Both KD and MIS-C can have cardiac sequelae, and KD is the most common cause of acquired heart disease in paediatric populations across the globe. Coronary aneurysm can be a potential complication in both KD and MIS-C. Left ventricular dysfunction is the most common cardiac complication of MIS-C, followed by coronary artery aneurysm and conduction abnormalities, which emphasises the need for different management and follow-up strategies based on cardiac involvement.7,8 Echocardiogram is recommended to screen coronary aneurysms and to assess ventricular dysfunction during the acute illness. Development of new coronary artery aneurysms can be seen in the convalescent phase of illness and serial follow-up echocardiograms at 1–2 weeks and then at 4–6 weeks are needed, even in patients with no cardiac abnormalities in the acute phase of illness.7 In addition, serial monitoring with B-type natriuretic peptide, troponin, and ECG is also recommended.
MANAGEMENT
Similarities and dissimilarities exist in management as well. Intravenous Ig (IVIG) with high-dose aspirin is the recommended first-line treatment of KD.5 Infliximab, cyclosporine, glucocorticoids, and plasmapheresis have been reported to be successful in treating IVIG-resistant cases. Antiplatelet and anticoagulation therapies should be considered for large (≥8 mm) and/or persistent coronary aneurysms. So far, there are no standardised treatment guidelines developed for MIS-C management, and low-dose aspirin is recommended to decrease the risk of thrombosis; long-term use of aspirin is tailored according to thrombophilic risk factors. Inotropic agents are used in MIS-C management due to diastolic dysfunction. Some observational studies have reported favourable outcomes with glucocorticoids in addition to IVIG as initial treatment, when compared to IVIG alone.7-9 However, to date, there are no available clinical trial data comparing these two treatment modalities. Clinical trials comparing infliximab, a TNF inhibitor, as an initial treatment along with IVIG compared to IVIG alone are emerging, and preliminary results suggest that patients treated with combination therapy are less likely to require additional therapy with vasoactive agents, had decreased length of intensive care unit stay, decreased development of left ventricular dysfunction, and more rapid decline in C-reactive protein levels.10 However, the data available so far are limited to the management of the acute phase of the disease, and no data are available regarding the long-term consequences of this disease.
The aetiology of KD remains unknown, and no preventative strategies are known. However, MIS-C is clearly related to SARS-CoV-2, and vaccinations for SARS-CoV-2 have been approved for children aged ≥5 years in many countries.11 Emerging research suggests that children as young as 6 months old might need to receive their own vaccine, as antibodies acquired passively by placental transport start declining at 6 months of age; there are ongoing trials regarding the safety and efficacy of SARS-CoV-2 vaccine in children 6 months and above.12 Further, recent studies have revealed that MIS-C is less common in children aged 12–18 years who were vaccinated with two doses of BNT162b2, and MIS-C could be a vaccine-preventable disease.13
CONCLUSION
KD and MIS-C have clinically overlapping features, but are different entities (Table 1). Early diagnosis and management are crucial for successful and timely management of both these conditions and follow-up. There is no standardised treatment available for MIS-C at this time, and further clinical trials are needed to compare the safety and efficacy of the various available treatment regimens, along with the effect on long-term outcomes. There is growing evidence that the incidence of MIS-C is low in fully vaccinated children, which further emphasises the importance of vaccination in children.
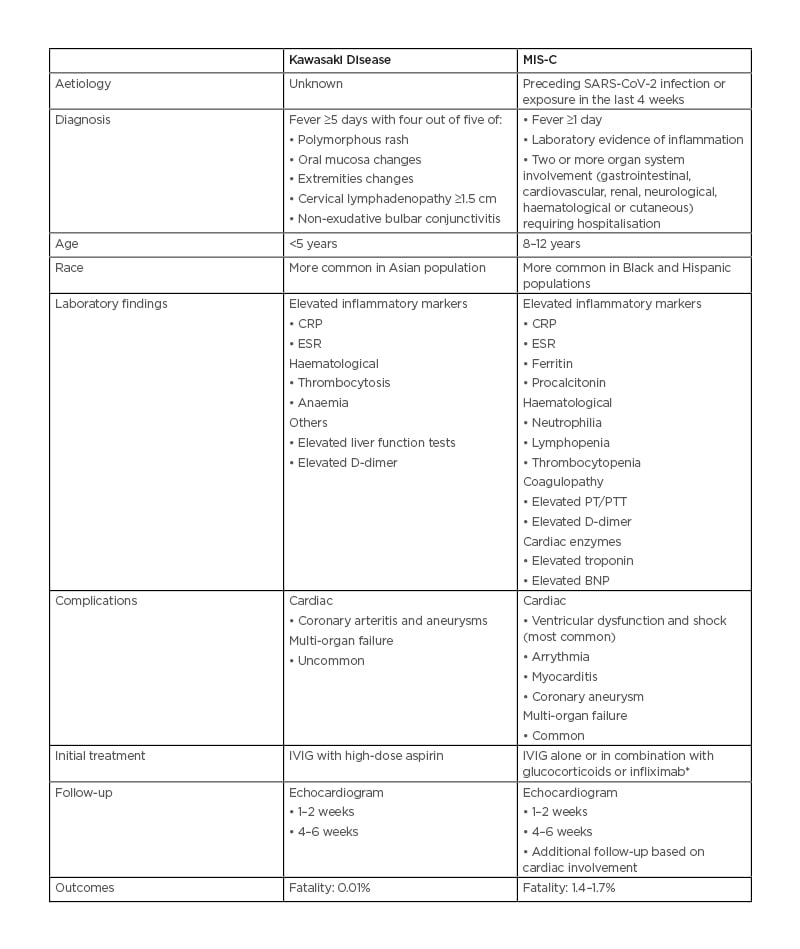
Table 1: Comparison between multisystem inflammatory syndrome in children and Kawasaki disease.
*Based on early clinical trial data.
BNP: B-type natriuretic peptide; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; IVIG: intravenous Ig; MIS-C: multisystem inflammatory syndrome in children; PT: prothrombin time; PTT: partial thromboplastin time; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.







