Abstract
Metabolic syndrome is a cluster of health conditions linked to increased cardiovascular disease. It is found worldwide in increasing proportions due to the modern lifestyle. The increase is visceral fat leads to secretion of harmful proinflammatory cytokines that have deleterious effects on various tissues, chiefly the heart and vasculature. Rheumatoid arthritis is a systemic inflammatory disease that shares pathogenic mechanisms with the metabolic syndrome. Patients with rheumatoid arthritis suffer increased heart disease over and above traditional risk factors. They have an increased occurrence of metabolic syndrome that enhance the risk further. Metabolic syndrome occurs early in the course of rheumatoid arthritis, creating clinical opportunities for prevention and control. Patients with both conditions also have more severe disease, pain, poorer functional status, less remission rates, and suboptimal response to treatment. Treatment of metabolic syndrome should be aggressive, using a proactive approach. Lifestyle measures are a corner stone, and this should be coupled with optimal control of rheumatoid arthritis, blood pressure, and lipid levels. The concerted efforts by a multi-disciplinary team of rheumatologists, primary care physicians, and other providers will set the stage for reducing the increased cardiovascular morbidity and mortality in these two conditions. More prospective studies are the need of the hour in determining the roles of the risk factors and the effects of lifestyle changes and medications in reducing the impact of the metabolic syndrome and its contribution to the already burdened pathology of rheumatoid arthritis. This narrative review discusses the latest in the field and identifies the areas that need further research.
Key Points
1. An increase in visceral fats associated with metabolic syndrome leads to secretion of pro-inflammatory cytokines, with harmful effects on various tissues, including the heart and vasculature.2. Overlap between rheumatoid arthritis and metabolic syndrome may result from shared pathogenic mechanisms. Patients with both conditions have more severe disease, pain, poorer functional status, lower remission rates, and suboptimal response to treatment, and particularly experience greater rates of atherosclerotic cardiovascular disease.
3. Screening for and treating metabolic syndrome are needed to reduce its impact on the pathology of rheumatoid arthritis and reduce the risk of cardiovascular disease associated with the pair of conditions.
INTRODUCTION
In 1988, Gerald Reaven coined the term Syndrome X. Reaven demonstrated that, in this syndrome, insulin resistance had a central role in increasing the risk of developing a cluster of conditions in people without diabetes. This cluster includes hypertriglyceridaemia; low high-density lipoprotein cholesterol (HDLc); small low-density lipoprotein (LDL) particle size; increased remnant lipoprotein; higher sympathetic nervous system activity; enhanced salt sensitivity; high plasminogen activator inhibitor-1; essential hypertension; and elevated serum uric acid.
Since then, the definition of this syndrome has undergone metamorphosis time and again. Subsequently, since insulin resistance was not present in all such instances, it was termed Metabolic Syndrome (Met-S).
Met-S has a higher incidence of atherosclerotic cardiovascular disease (ASCVD) and Type 2 diabetes (T2D), and consequent morbidity and mortality. T2D is an independent risk factor for ASCVD. Therefore, it is a priority for healthcare providers, policymakers, and patients to take appropriate measures to prevent and treat the condition. Various organisations such as the World Health Organization (WHO), International Diabetes Foundation (IDF), European Group for the study of Insulin Resistance (EGIR), American Association of Clinical Endocrinologists (AACE), and National Cholesterol Education Program’s Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (NCEP-ATP-III) have issued their criteria to define this condition resulting in differences in the reported prevalence of Met-S. The Endocrine Society practice guidelines 2019 have introduced ‘Metabolic Risk’ instead of ‘Metabolic Syndrome’. Moving away from attempting to define a constellation of findings to qualify the Met-S to one of the identifying risk factors that increase ASCVD and T2D risks is welcome. It will help the practitioner look outside the box and examine traditional risks like family history of CVD, smoking, and LDL cholesterol, which are not part of the Met-S criteria. The European League Against Rheumatism (EULAR) has recommended a similar approach but with some differences.1,2 Notwithstanding, the Met-S is still useful from a practice point of view (Tables 1 and 2). The presence of obesity is not a prerequisite for diagnosing Met-S because some patients who are not obese have the condition (metabolic obesity), and some are obese but do not have this conditon.3 There is not much difference in the relative contribution of each of the components to Met-S. The risk for ASCVD persists even after eliminating T2D from the list. There is debate on the role of T2D in increasing this risk. We do not yet know whether the sum of the components is more than the individual components as far as the prognosis of Met-S is concerned.
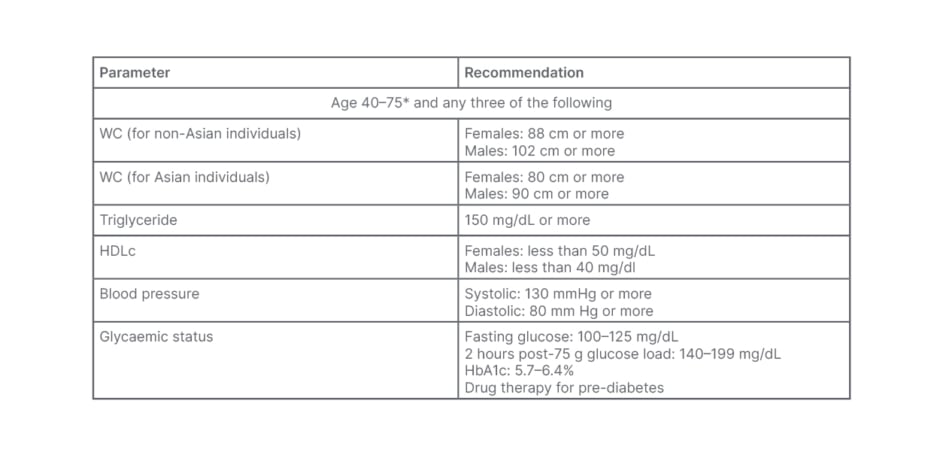
Table 1: Endocrine Society criteria for metabolic syndrome in Asian and non-Asian populations.
*May evaluate patients younger than 40 years of age if clinically indicated.
HbA1c: glycated haemoglobin; HDLc: high-density lipoprotein-cholesterol; WC: waist circumference.
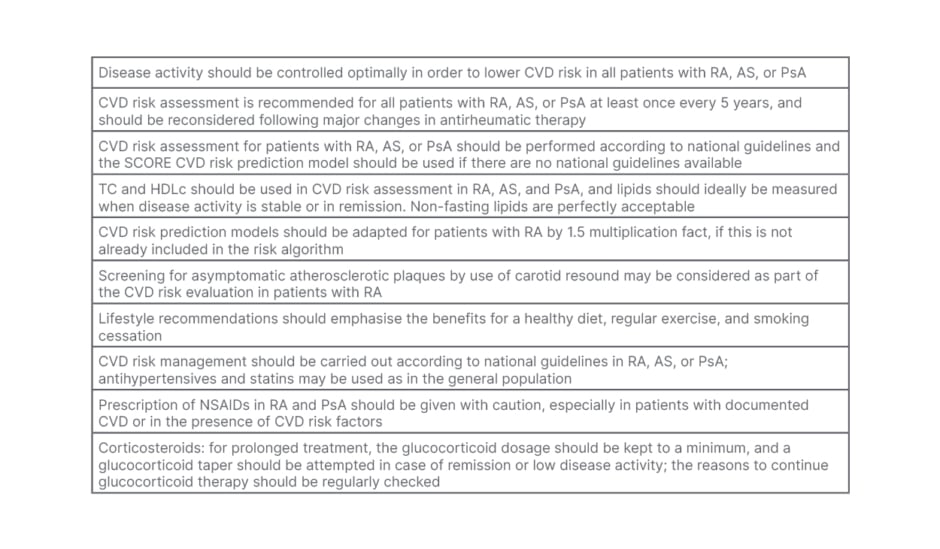
Table 2: European League Against Rheumatism (EULAR) criteria for cardiovascular risk assessment in rheumatoid arthritis and other inflammatory arthritides.
AS: ankylosing spondylitis; CVD: cardiovascular disease; HDLc: high-density lipoprotein-cholesterol; NSAID: non-steroidal anti-inflammatory drug; PsA; psoriatic arthritis RA: rheumatoid arthritis; TC: total cholesterol.
ARE THERE OTHER MARKERS THAT PREDICT RISK?
Apart from the above, many other factors that depict ASCVD and T2D risks are receiving attention, and may be significant in some subpopulations. Still, there is insufficient evidence for their routine use in calculating ASCVD risk (Table 3).
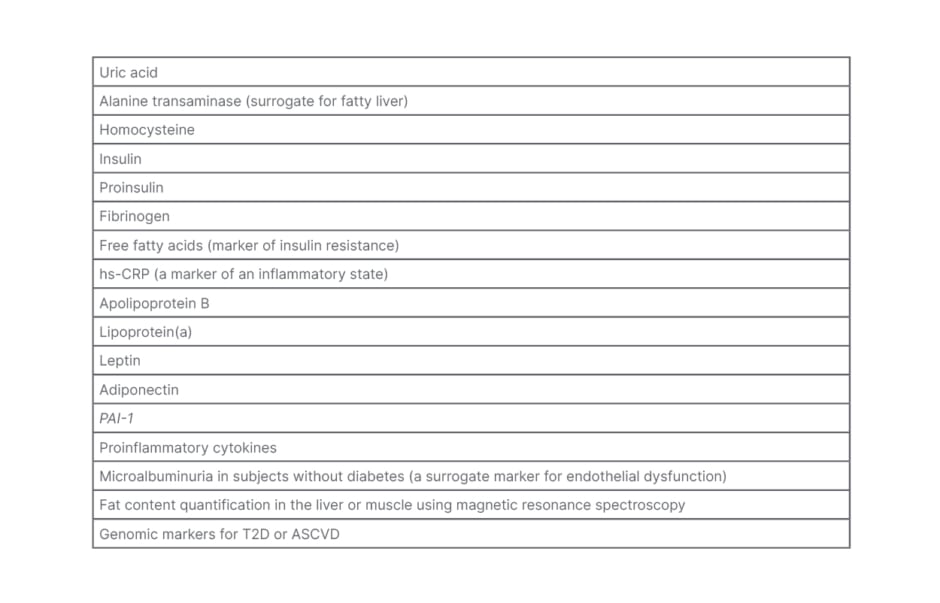
Table 3: Other markers that could predict risk.
ASCVD: atherosclerotic cardiovascular disease; hs-CRP: high-sensitive C-reactive protein; T2D: Type 2 diabetes.
PREVALENCE OF METABOLIC SYNDROME
The prevalence of Met-S varies in different populations using different criteria. It can be explained by ethnic factors, as reported in many studies. The Asian populations have more fat for a given BMI. The upper limit of normal BMI in this population is 23 kg/m2, and obesity is a BMI of 27 kg/m2 or more. A waist circumference of 90 cm for men and 80 cm for women correlate with a visceral fat area of 100 cm2. The visceral fat area values above 100 cm2 have one or more obesity-related disorders such as hyperglycaemia, hypertension and dyslipidemia.4-6 By using western norms, many Asian people will be branded as normal when they have Met-S. In a significant study, patients with rheumatoid arthritis (RA) had more fat for a given BMI than normal, similar to Asian people. They proposed a cut off of 23 kg/m2 and 28 kg/m2 for patients with RA who are normal and obese, respectively.7
Further testing is required in more patients with RA, but it does explain the result of a chronic inflammatory process.
WHAT CAUSES METABOLIC SYNDROME?
Lifestyle is key. A diet high in sugar, refined flour, red meat, and fats is causative, while a Mediterranean diet appears protective. Smoking is a risk factor that increases with the number of cigarettes smoked. Excess alcohol consumption also increases this risk. Physical inactivity increases body weight and BMI, enhances visceral fat deposition, alters lipid levels, decreases insulin sensitivity, and elevates blood pressure (BP). Genetic and epigenetic factors also influence these changes.
Visceral adipose tissue (VAT) is a metabolically distinct endocrine organ. It secretes several adipokines and cytokines. Adiponectin and omentin are anti-inflammatory, enhance insulin sensitivity, and modulate immune functions. In RA, adiponectin levels are reduced during active disease and correlate inversely with total cholesterol or HDLc index, blood glucose, and high-sensitivity C-reactive protein (hs-CRP) levels. This association is independent of BMI and hs-CRP levels and promotes atherogenesis.8 IL-1β, IL-6, IL-8, TNF-α, MCP-1, and lipocalin-2 are pro-inflammatory. Excess VAT accumulation produces an imbalance in cytokine production favouring insulin resistance, elevated BP, adversely altered quantity and quality of lipid levels and consequently increases cardiovascular disease (CVD) risks. Manipulation of these cytokines is an attractive therapeutic target in managing Met-S.
RHEUMATOID ARTHRITIS AND METABOLIC RISK
RA and Met-S have inflammation as a core component in their pathogenesis. RA bites both the joints and the heart, whereas rheumatic fever licks the joints but bites the heart.
Met-S is higher in RA than in the general population in most studies.9,10 However, studies from Mexico and Iran showed no differences in the former, and lower prevalence in the latter.11,12 This may have been due to selection bias, medication effects, and other factors. A study from Singapore showed no change in outcomes in RA with Met-S, but revealed ethnic differences in CVD risks.13 A study from Turkey revealed the presence of Met-S in naïve early RA.14
A study from North-East India included many treatment-naïve patients who had Met-S.15 This confirms a common pathogenic pathway for both conditions and opens a window of opportunity for early intervention. In the KNHANES study, Met-S was lower in treated RA.16
Features of Metabolic Syndrome in Rheumatoid Arthritis
RA with Met-S has more dyslipidaemia and glucose abnormalities. Both RA with CVD and RA without overt CVD have Met-S. The latter have evidence of subclinical atherosclerosis as a result of increased carotid artery intima-media thickness (cIMT) and characteristic findings in cardiac MRI.17-19 Met-S is higher in longstanding RA, individuals who are older, smokers, males, and post-menopausal females, and those with higher Disease Activity Score-28 (DAS-28) scores.20,21 Males with RA and Met-S had higher individual components of the Met-S than females. Patients with RA and Met-S had lower vitamin D levels.22 The coronary artery calcium scores are higher in RA, and Met-S aggravates this.
Patients with both conditions have more pain, increased physical inactivity, poorer functional status, and resistance to traditional treatments. Met-S added to RA leads to lower remission rates.23
Cardiovascular Risks in Rheumatoid Arthritis
Only a quarter of patients with RA received antihypertensive treatment for hypertension; among those patients, only half had optimal control of systolic BP until a few years ago. The situation has changed, and many patients with RA and hypertension now receive optimal treatment. Elevated hs-CRP, glucocorticoids, and non-steroidal anti-inflammatory drugs aggravate hypertension. Hypertension increases mortality from myocardial infarction by 80% in patients with RA. RA independently raises the risk for ASCVD, as diabetes does. The EULAR guidelines advise that the ASCVD risk calculated from traditional risk calculators be multiplied by 1.5 for patients with RA.
Since the conventional risk factor algorithms underestimate the risk for ASCVD in RA, there is a role for non-invasive tools like cIMT measurements using carotid ultrasound for risk assessment (Table 2).24 This risk is over and above that contributed by conventional factors. So, patients with RA have double jeopardy. As noted earlier, Met-S is higher in RA and is detected even in the early stages.
CVD in RA goes beyond the traditional risk factors. Patients with RA have a 50% increase in CVD, and almost twice as many acute coronary events and strokes as the average population with similar traditional risks, as shown in the Nurses’ Health Study. In some studies, CVD risks predate the first joint symptoms of RA. In patients with RA who have cardiac symptoms but negative non-invasive tests, cardiac MRI reveals abnormalities in cardiac function. Burggraaf et al.25 followed RA patients both with and without Met-S, and found that treatment for RA did not prevent the subclinical progression of atherosclerosis as measured by cIMT. This not only shows the aggravating role of Met-S in RA, but also that treatment of RA alone is insufficient, and that Met-S has to be dealt with individually. When T2D and RA occurred together, ASCVD was double, and markers of Met-S (hyperlipidaemia, hypertension) increased.26
Biomarkers Contributing to Enhanced Metabolic Syndrome and Cardiovascular Disease in Rheumatoid Arthritis
IL-6, IL-1β, and TNF-α are key in RA and ASCVD.
TNF-α increases dyslipidaemia, enhances oxidised LDL, enhances foam cell formation, impairs the antioxidant effects of high-density lipoprotein (HDL), and compromises endothelial integrity. It reduces nitric oxide and thrombomodulin and promotes coronary calcification and a prothrombotic environment.
IL-1β increases the expression of adhesion molecules that promote atherosclerosis.
Other contributors to increased morbidity include increased oxidative stress, citrullination of LDL rendering it more atherogenic, activation of the cluster of differentiation 40–cluster of differentiation 40L axis pathways, which enhance inflammation and fibrosis and lead to plaque instability, rupture, thrombosis, and coronary events.
Serum amyloid A is high in RA, binds to HDL, decreases its anti-oxidative functions, and promotes ASCVD.
Nuclear factor κ-light-chain-enhancer of activated B cells is key to inflammation in RA. It elevates IL-6, IL-17, IL-18, IL-33, TNF-α, hs-CRP, vascular cell adhesion protein 1, and intercellular adhesion molecule 1. Many of these stimulate multiple pro-inflammatory pathways simultaneously and produce a synergistic effect that is far greater than the sum of their contributions. The results are devastating atherogenesis and thrombosis.27
Epicardial fat is VAT, increased in RA with Met-S. Research has shown that increased epicardial fat volume produces dysfunctional adipocytes that secrete pro-inflammatory cytokines that promote atherosclerosis and coronary calcification.28 Higher intermuscular and intramuscular fat correlated with levels of VAT and Met-S.29
Assessment of risk for Metabolic Syndrome in Rheumatoid Arthritis
Subclinical atherosclerosis demands a proactive approach to Met-S to prevent CVD events and silent myocardial infarction. Screening for Met-S should be routine in the medical evaluation of patients with RA. RA specific CVD risk assessments are available. A Korean pilot study found the Expanded Risk Score in Rheumatoid Arthritis (ERS-RA) helpful in predicting subclinical atherosclerosis. However, a validation analysis of data from seven countries using several RA specific risk scores for estimating CVD risks did not find any superior methods to traditional risk scoring systems. In surveys of practising rheumatologists in the USA, <40% addressed CVD risks in their patients. This gap has to be closed to achieve any meaningful decrease in CVD. An alternative to this is the education and involvement of primary care providers. Another alternative is a multi-disciplinary clinic. It should comprise rheumatologists, endocrinologists, cardiologists, radiologists, and primary care providers with provider-specific tasks.
Individual Tests for Metabolic Syndrome
Anthropometry
A waist circumference (WC) is measured (Table 1). The WC is a useful marker for visceral adiposity and Met-S. It is easy to perform in the physician’s office and takes very little time. If accurately measured, it will ease risk classification and intervention. Alternatively, an index derived from the WC divided by height is also useful and has a good correlation with markers of Met-S. It has various terms; the index of central obesity, weight-to-height ratio, or weight-to-stature ratio, and the normal cut off is 0.5. Other measurements like the visceral adiposity index and the lipid accumulation product are yet to be validated.30-32
Blood pressure measurements
Standard protocols for accurate measurements of BP remain valid when evaluating patients with RA and Met-S (Table 4).
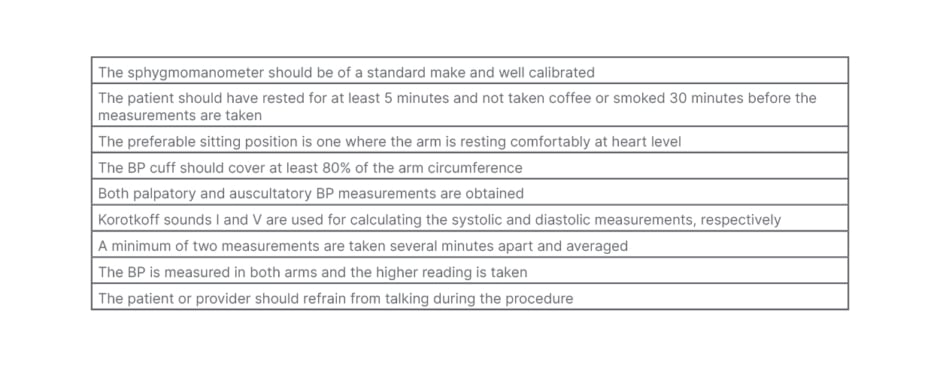
Table 4: Standard protocols for taking accurate blood pressure measurements.
BP: blood pressure.
Lipid profile
A fasting sample will yield the total and HDL cholesterol and triglyceride (TGL), which vary in the EULAR guidelines.
Glycaemic status
Fasting glucose, a two-hour post 75 g glucose load test, or an HbA1C can be used, which varies in the EULAR guidelines.
If three or more components of Table 1 are detected, the height, weight, BMI; the LDL cholesterol, and non-HDLc are measured.
If the TGL is more than 400 mg/dL, the calculated LDL is inaccurate and LDL is directly measured.
The patient should be screened for smoking, a family history of ASCVD, and be enrolled in a programme for lifestyle modifications (Table 5).
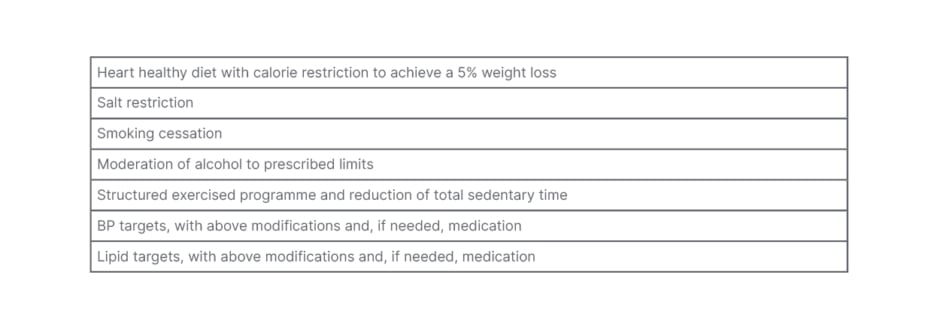
Table 5: Lifestyle modifications
BP: blood pressure.
For those who have only one or two components of Table 1, after the additional screening, lifestyle modifications are done as above, and periodic monitoring in intervals of 1–3 years is mandatory depending on circumstances.
WHAT FACTORS ARE SPECIFIC TO METABOLIC SYNDROME IN RHEUMATOID ARTHRITIS?
The above protocol is applicable in patients with RA with Met-S, but existent studies are cross-sectional, and evidence-based studies are lacking. There are handicaps in RA that may interfere with the smooth implementation of a lifestyle program. Active disease may cause fatigue, pain, restricted joint mobility, and in later stages, deformities. Therefore, it is imminent that effective treatment of RA and Met-S management must go together. A pain-free joint with a good range of motion will make an exercise programme most effective. However, all is not lost if handicaps already exist. It is where experts in physical medicine can be of help in structuring an individualised exercise program. An example is water aerobics for patients who experience pain and restricted mobility when doing regular exercises.33
The disease activity or treatment of RA with particular agents may alter the values of the glucose or lipid measurements. It will not only change the Met-S classification status, but may render the treating physician to be lax on instituting measures to prevent or treat ASCVD. An example is a patient in an inflammatory state with decreased LDL, HDL, and TGL levels (lipid paradox) who fails to qualify as Met-S. The same patient will meet the criteria for Met-S when assessed after treatment once the inflammation has reduced and lipid levels have risen to their ‘true’ values. Treatment with hydroxychloroquine (HCQ) decreases glucose levels and RA disease activity. A patient with RA under treatment with HCQ will not meet the criteria for Met-S during treatment. In such instances, the risk for coronary events remained elevated despite normal lipid levels. An elevated hs-CRP, which is a risk for ASCVD, could cause decreased lipids. Studies from different sites have revealed varied conclusions.
LIFESTYLE MODIFICATION AND ITS IMPACT ON RHEUMATOID ARTHRITIS OUTCOMES
Smoking
It is well established that smoking increases CVD in the general population. In RA, smoking increases antibody production (rheumatoid factor, anti-citrullinated antibody), worsens the disease, diminishes gains from therapy, and increases ASCVD morbidity and mortality. Smoking cessation should receive top priority to reduce RA activity and ASCVD. García-Chagollán et al.34 showed that in RA with Met-S, smoking correlated positively with higher disease activity, higher levels of hs-CRP, and RF yielding evidence that treatment of Met-S can decrease RA and ASCVD.35
Exercise, Weight Loss, and Dietary Patterns
Physical inactivity is inversely related to CVD events and increasing physical activity decreases CVD deaths significantly. Patients may have additional handicaps such as pain, fatigue, joint stiffness, deformities, and inertia that hinder an exercise programme. Successful exercise programmes in patients experiencing these symptoms result in significant improvements in BP, lipids, hs-CRP, homocysteine, plasminogen activator inhibitor-1, and endothelial function, apart from musculoskeletal benefits.36 Improved cardiopulmonary fitness using aerobics and resistance will aid in successful participation in an exercise programme and promote quality of life.37
A heart-healthy diet goes hand-in-hand with an exercise programme. Three landmark studies (Da Qing study, Finnish Diabetes Prevention Study, and Diabetes Prevention Program) on the effect of diet, weight loss, and increased physical activity in patients with elevated metabolic risk (Met-S) consistently showed improvements in BMI, WC, BP, lipids, hyperglycaemia, and ASCVD. More intense exercise and calorie restriction had improved outcomes. Dietary patterns (Mediterranean style, which has anti-inflammatory effects reducing pro-inflammatory cytokines) and salt restriction showed superiority to conventional western diets. More importantly, the results of such interventions were persistent even many years after the interventions, confirming ‘metabolic memory’.38-40
Although these results were on patients without RA, they can extrapolate to RA.
Walrabenstein et al.41 reported favourable outcomes of a whole food plant diet in RA. Based on encouraging results, they outlined a prospective extension study integrating exercise and stress reduction with whole food plant diet for two years on the natural history of established (Arm-1) and new (Arm-2 in patients who are anti-citrullinated antibody-positive without clinical disease) RA. The results will answer many questions on the roles of lifestyle interventions in RA, ASCVD, and Met-S.41
MEDICATIONS AND EFFECTS ON RHEUMATOID ARTHRITIS AND ATHEROSCLEROTIC CARDIOVASCULAR DISEASE OR METABOLIC SYNDROME
Lower disease activity in RA decreases ASCVD risk factors and CVD events. With TNF-α inhibitors, optimal disease control decreases ASCVD significantly, but symptom control alone does not. This agent improved insulin sensitivity, decreased oxidised LDL, improved pulse wave velocity, reduced arterial stiffness, and reduced myocardial infarction significantly.42
Methotrexate is often the first-line therapy for RA. It decreases harmful cytokines, improves the antioxidant function of HDL, prevents foam cell activation, enhances the scavenging of free radicals, improves insulin-mediated glucose transport, and restores endothelial function. Met-S was lower in RA treated with methotrexate.43 There is a 20% reduction in the composite CVD event rates from pooled data from ten studies.44
HCQ has many benefits in RA. It improves insulin sensitivity and lipid profile. It suppresses IL-1, IL-6, and TNF-α production. It prevents the proliferation of T lymphocytes and toll-like receptors, enhances endothelial function, creates an antithrombotic milieu, and consequently reduces ASCVD.42,45,46
The IL-6 inhibitor tocilizumab makes HDL more anti-atherogenic. In addition, it improves insulin sensitivity, even in patients with RA but without diabetes.47,48
There were concerns about the LDL increase with tofacitinib (a JAK inhibitor). The STAR-RA trial recently advised caution in patients with RA and CVD risks.
Many studies have benefits of statins in RA. Atorvastatin fared better than simvastatin, showing a reduction in LDL, TGL, and hs-CRP; an increase in HDL; and improvement in DAS-28 scores. The pleiotropic effects of the statins effectively reduced RA activity and ASCVD risk.49
The deleterious effects of glucocorticoids are well known. Although they reduce inflammation, they cause and perpetuate Met-S: a higher dose and longer duration are associated with more side effects. The EULAR guidelines advise restricted use.
CONCLUSION
Patients with RA are at high-risk for ASCVD. This risk is well above the traditional risk factors.50 Met-S is increased in RA and contributes to a higher disease burden. It occurs early in its course and is associated with less remission. It is higher in males and post-menopausal females, with older age, smoking, increased disease activity, and suboptimal treatment. Subclinical atherosclerosis is a hallmark of RA, and Met-S aggravates it. RA and Met-S have common inflammatory aetiologies. Patients with should be screened for metabolic risk early, during initial diagnosis and should look outside the Met-S box. Treatment of individual metabolic risk factors is in addition to optimal control of RA. Hypertension in RA worsens ASCVD risk and events and is not well managed currently by practitioners, especially in developing countries. Many anti-rheumatic agents reduce ASCVD and the contributing factors to Met-S. Lifestyle modification is the first line of management of Met-S in RA. More prospective studies examining the specific role of Met-S in RA and the means to reduce it are needed. The education of primary care providers on the optimal care of Met-S in RA and the institution of multi-disciplinary clinics are the need of the hour.







