Abstract
Chronic inflammatory diseases (CID) share many common features, such as debilitating illness, increased mortality, impaired quality of life and productivity, and high economic burden. The approach to treating CID has shifted over the last 20 years from symptom to mechanism of action-targeted therapy following the development of primarily biologic drugs, in which the same therapy can potentially treat multiple diseases. Developing these drugs requires novel strategies and a multidisciplinary approach for implementation. This article provides an overview of shared features for CID clinical trials and addressing common challenges in their planning and execution. Since CID studies often test the same drug for treating different pathologies, knowledge of the drug from previously investigated therapeutic indications can be leveraged when planning clinical trials. Given the variety of CID signs and symptoms, eligibility criteria need to clearly define the target patient population by minimising ambiguity and risk of misunderstanding. Other common challenges include an elevated response in the placebo arm, the subjectivity of investigator assessments, and the use of appropriate patient-reported outcomes. Several measures can help minimise the impact of the aforementioned issues on study outcome, including centralised eligibility review and endpoint adjudication, tight control of background therapy and concomitant medications, and intensive training of assessors. The above common features support an approach to CID as a largely interconnected therapeutic area in which a multidisciplinary approach, application of common strategies, and lessons learnt across different indications represent crucial factors for effectively planning and executing clinical trials.
INTRODUCTION
Chronic inflammatory diseases (CID) encompass a wide range of pathologies affecting different organs or bodily functions. Besides signs and symptoms specific to the organ or the function, they all present common features, such as a long-lasting and debilitating illness, increased mortality, impaired quality of life and productivity, and high social and economic burden.
Most CID are characterised by a dysregulation of the innate or adaptive immune system, leading to and perpetuating a chronic inflammatory condition. Their estimated prevalence varies from 5% to nearly 10%, and is increasing.1,2 A systematic review to identify the incidence and prevalence of immune-mediated inflammatory diseases over the period 1985–2015 found mean net percentage increases per year (standard deviations) of 19.1±43.1 and 12.5±7.9, respectively.3 Among the most prevalent CID, psoriasis accounts for >120 million cases worldwide, while rheumatoid arthritis and psoriatic arthritis amount to >70 million cases each.4,5,6
In the following sections, an overview of the main features shared by clinical trials of CID is provided, highlighting challenges and potential mitigation.
Since, as stated initially, a huge number of pathologies can be classified as CID spanning across almost all therapeutic areas, it is beyond the scope of this review to cover all of them. While the following discussion will focus primarily on select CID within rheumatology, dermatology, and gastroenterology, the majority of the identified features and challenges can also apply to other disease areas.
CHALLENGES AND COMMON FEATURES OF CHRONIC INFLAMMATORY DISEASES CLINICAL TRIALS
One Drug for Multiple Indications
Since the introduction of the first TNFα inhibitor for the treatment of rheumatoid arthritis, the strategy of CID management has focussed on the identification of altered inflammatory or immune pathway(s) and the search for related targets for drug development. The approach to treating CID has substantially shifted from symptom to mechanism of action-targeted therapy.7 As a result, the same drug can potentially treat multiple diseases, as the same immune or inflammatory pathway can be shared by different pathologies.
A crucial role in the pathogenesis of CID is played by several proinflammatory cytokines such as TNF, IL-6, or interferon γ. These are considered pleiotropic cytokines, meaning that they show multiple biological actions and, therefore, they can represent an optimal target for drugs directed to treat multiple CID. 8,9 TNF-inhibitors are currently approved for treatment of several rheumatologic, skin, and gastrointestinal autoimmune CID. Similarly, IL-6 inhibitors are presently approved to treat various rheumatologic diseases, giant cell arteritis, and Castleman’s disease, and are being tested for other types of CID. New cytokines have more recently emerged as targets for multiple CID, for example IL-17 or IL-23.10 Other targets that have proved adequate to treat multiple CID are intracellular signal transducers such as those of the JAK or signal transducers and activators of the transcription family.11
Planning the development of these new classes of drugs that are either monoclonal antibodies or small molecules has required novel strategies. These include upfront selection of the potential CID to treat with a choice of therapeutic indications to pursue for first marketing authorisation, or a choice of the most adequate clinical model for a preliminary demonstration of the drug’s biological efficacy. Implementation of such development strategies in the early clinical phases with possible subsequent adjustments require a multidisciplinary approach, including feasibility assessments, market analyses, and clinical development expertise in multiple therapeutic areas. A multidisciplinary approach with sharing experience across different therapeutic indications also proves helpful at the time of planning and executing clinical trials because of the common features they present, as this review discusses in greater detail.
Treatment-Associated Features
Because the same drug is tested to treat different pathologies, knowledge of the drug from previously investigated therapeutic indications can be leveraged when planning clinical trials. For example, even if differences exist among different patient populations receiving the same product, knowledge of drug-related side effects, pharmacokinetic characteristics, immunogenicity in the case of biological drugs, administration modalities, and pharmacodynamic markers all contribute to increase a multidisciplinary team’s familiarity with the study drug and facilitate planning and execution of clinical trials.
In addition, patients enrolled in CID trials are often receiving background standard of care with immunosuppressive drugs such as methotrexate, azathioprine, mycophenolate mofetil, or with corticosteroids. Knowledge of side effects and precautions associated with the use of these treatments can be also shared across different therapeutic indications to help design adequate study protocols.
Disease and Patient Characteristics
Different CID, even if affecting primarily a specific organ or bodily function such as the joints or skin, often present with a variety of common signs and symptoms that involve the entire body, such as fatigue, fever, anaemia, myalgia, arthralgia, and ocular or renal symptoms. A patient can also suffer from a combination of different CID or develop different CID over time. Moreover, each of these diseases can present a wide spectrum of severity. Medical management can also greatly vary across geographies, depending especially on access to novel therapies and local reimbursement policies.12,13
Therefore, it appears evident that enrolling a relatively homogeneous patient population with a definite diagnosis may be challenging. Special attention is to be paid to designing a study protocol with eligibility criteria that clearly define the target patient population by minimising ambiguity and risk of misunderstanding. Eligibility criteria should include severity of disease assessed by standardised methods, a list of concomitant pathologies that are exclusionary, and allowed or prohibited concomitant medications with pre-defined minimal washout duration. On the other hand, when defining such criteria, evolving standard of care is to be taken into account to be able to achieve recruitment goals. For example, while enrolling biologic-naïve patients is becoming more difficult, even in regions with poor access to expensive therapies, it may be more feasible to establish a limit to prior exposure to biologics (e.g., not more than one previous biologic agent) to facilitate recruitment and at the same time avoid recruiting subjects with refractory disease.
Conducting a survey assessment across potential investigational sites on the main features of the protocol and consultation with key opinion leaders is crucial to get a clear picture of the feasibility of the study protocol and recruitment capability.
Patients with CID are commonly known by the clinics taking care of them, given the chronic nature of their condition. This means that study sites have a database of patients, from which they can pre-select subjects who could be eligible for trial participation and can therefore plan for enrolment of patients in a timely fashion and in co-ordination with their clinical activity. In the authors’ experience, patients who have a long-standing relationship with a study site also tend to be more compliant with protocol requirements and to remain in the study until completion.
In addition, there are well-organised advocacy groups for most CID, which can be leveraged for disseminating information on the clinical trial and facilitate recruitment.
Placebo Response
One of the major challenges and reasons for study failure in CID is an elevated response rate in the placebo arm. Many factors can contribute to increased placebo response in clinical trials, some of which are especially important for CID. They include subjectivity of disease assessments at screening and in the course of the study, and the need for background therapy in all study participants14-17. This last point is of particular interest for CID that often require keeping all patients enrolled in a clinical trial on a stable background therapy, given the chronicity and the severity of the condition. It is well known that patients with such chronic diseases tend to show poor compliance with therapy. But their compliance usually increases when enrolled in a clinical trial and therefore they are subject to closer medical control. As a result, the effectiveness of their background therapy improves together with the response to placebo given on top of it.
In rheumatology, dermatology, or gastroenterology indications of CID, composite indices of disease activity are often used that heavily rely on assessments performed by the investigator or the patient. In rheumatoid arthritis, count of swollen and tender joints, physician assessment of disease, patient assessment of disease and pain, and health assessment questionnaire are all evaluations that are heavily dependent on individual experience or the subject’s perception of their own condition. Two recent studies have shown how these assessments can impact placebo response. In a meta-analysis of 165 randomised controlled trials in rheumatoid arthritis, a placebo effect size for pain relief of 0.28 (95% confidence interval: 0.19, 0.37) was found.14 Significant placebo effect size for other outcomes was also found, such as physician and patient disease assessment, tender joint count, swollen joint count, and function. Surprisingly, a meta-analysis of 10 Phase II or III randomised controlled trials in rheumatoid arthritis, which enrolled and treated with placebo on top of methotrexate nearly 1,000 subjects, found that assessments performed by investigator or site staff were more sensitive to placebo response compared to patient-reported outcomes (PRO).15
Several measures can help limit or at least keep under control placebo response: adequate training of site staff to standardise and make their assessments as objective as possible; training of patients on correct completion of PRO; introduction of a run-in period on background therapy prior to randomisation; preference for objective measures of disease, like blood tests analysed at a central laboratory, or central review of key data; consideration of the cultural or geographical differences (e.g., higher placebo response commonly reported from Latin America15); and realistic statistical assumptions for sample size estimates.
Selection of appropriate patients as per protocol is also crucial to reduce the risk of elevated placebo response. Enrolling subjects with a lower than required disease severity is a common issue for CID trials, despite well-defined eligibility criteria.18 Close control of screening procedures and possibly implementation of a central eligibility review can help. Traditionally, a central eligibility review is implemented in systemic lupus erythematosus (SLE) trials18 wherein the risk for misclassifying disease severity at study entry is high. For example, the majority of SLE trials require a certain level of disease severity based on a composite index called Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), which is also an important component of the study primary endpoint. The SLEDAI is based on the assessment of various symptoms or signs attributable to lupus, each of which is assigned an individual score, with the overall score being the sum of the individual scores. Petri et al.19 have shown that mistakenly reporting the presence of headache or vasculitis due to lupus, which both have a very high SLEDAI score, can erroneously inflate the screening and baseline total SLEDAI scores. In a post-hoc analysis of a Phase III study in SLE, the authors observed that the high placebo response could be partly explained by the ‘disappearance’ of headache or vasculitis in the course of the trial, which was having a strong impact on the patient’s response.19
Subjectivity of Assessments
As mentioned previously, the majority of CID trials are negatively impacted by subjectivity of assessments used to define the study efficacy endpoints. A few examples of such assessments for different pathologies are listed in Table 1, which is not intended to be exhaustive in terms of either diseases or assessments. Some of the assessments included in the table are a mix of objective and subjective (either from investigator or patient) evaluations, such as the Disease Activity Score-28, Crohn’s Disease Activity Index, or Mayo/Ulcerative Colitis Disease Activity Index score.
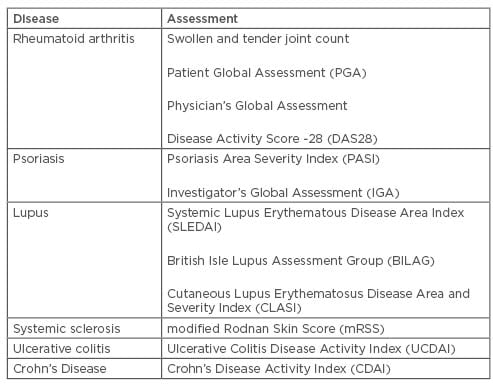
Table 1: Assessments of chronic inflammatory diseases.
Even assessments that appear to be objective because they are based on observation and physical examination, such as count of swollen and tender joints, have been shown to strictly depend on the assessor’s experience and employed methodology. Several studies have reported considerable variability among individual assessors or clinical sites for both tender and swollen joint count.20,21
Interobserver variability seems to be higher for smaller joints and for swollen joints compared to tender joints.19,22 For the Crohn’s Severity Activity Index, which is essentially based on a physician’s interpretation of patient symptoms, significant interobserver differences were noted in various studies.23 Other studies reported assessment reliability within a moderate-to-good range.24
The modified Rodnan Skin Score, an assessment of skin thickness, is commonly used in clinical trials of systemic sclerosis as a surrogate endpoint for disease activity and severity. It has been shown to exhibit a high interobserver variability.25
Because in almost all assessments discussed above the intraobserver variability tends to be significantly lower than the interobserver one, the first recommendation for CID studies is that key disease activity assessments are performed by the same assessor throughout the trial or at least within the same subject. All assessors need to be properly trained and qualified to standardise the method of assessment across study sites. Differences among assessors may be due not only to different levels of experience but also to differences in local practice and the adopted methodology. Training can be conducted by employing a combination of different tools, for example the use of video demonstration, Webex training, implementation of dedicated educational web portals, and/or live demonstration by qualified trainers at investigator meetings. It is recommended that trainees are required to pass a test at the end of a training session to receive certification of training. Training should also be tailored to each individual study.
When feasible and appropriate, central adjudication can be used so that the assessment for all patients enrolled in the study is performed by an adjudication committee. This is the case for the British Isles Lupus Assessment Group’s (BILAG) scoring index where usually centralised review and adjudication on an ongoing basis is implemented in clinical trials for lupus. The adjudication process is commonly supported by onsite review conducted by experienced study monitors and preprogrammed automatic edit checks to clean local assessments and correct inconsistencies.18
Central reading is also implemented for instrumental evaluations like endoscopy in inflammatory bowel diseases, spirometry in respiratory diseases, or joint radiography in rheumatology to guarantee standardisation and unbiased evaluation.
Biomarkers
In recent years, attention has focussed on the identification of biomarkers specific for individual or groups of CID.26,27 Besides being used to expedite initial proof of mechanism studies and screening of drug candidates, biomarkers can allow for the prediction of response to treatment or stratification of patients. As such, they can support the attempt to develop personalised medicine for CID, similarly to what is being done in oncology and other therapeutic areas. There are several examples of such biomarkers. Systemic lupus is a CID in which the search for biomarkers is particularly active due to the heterogeneity of disease and efficacy of drugs that apparently is limited to subgroups of patients.28,29 Among others, Type I interferon gene signature has emerged as a marker of disease severity and as a potential tool to stratify patients for tailored treatment.30
Therefore, incorporating biomarker evaluation into CID clinical trials from early to late phase has become a common practice.31
Patient Reported Outcomes
As recommended by regulatory agencies,32,33 use of PRO in clinical trials is increasing and also extended to early phase studies. In CID, PRO usually include evaluation of patient pain, global disease, functional limitation, and quality of life, among others. The same or very similar PRO are used across multiple pathologies. For example, the health assessment questionnaire that allows for assessment of functional limitations in daily activities due to the disease is commonly employed in all rheumatologic conditions.34 Fatigue is a typical symptom of CID that can become more pronounced during flares and can impact a patient’s functioning and quality of life. It is often evaluated by means of the Functional Assessment of Chronic Illness-Fatigue questionnaire. Several tools are available for quality of life evaluations. Some of these, such as the 36-Item Short Form Health Survey or the EuroQoL Research Foundation’s EQ-5D instruments, are generic and are employed across many different pathologies, while other tools are disease-specific. Depression scales, such as the Hospital Anxiety and Depression Scale, are also often administered because depression tends to be more frequent in subjects with CID.35,36 Pain scales can include the Visual Analog Scale, Numeric Rating Scale, or more specific tools. Some of the aforementioned scales or questionnaires can be administered during clinic visits, while others are to be completed daily in a patient’s diary.
When selecting PRO for a clinical trial, some practical aspects are to be considered upfront. First of all, the most appropriate device used for recording the assessment is to be chosen. Nowadays, electronic devices are preferred over the traditional paper diary or questionnaire; however, attention should be paid to the age of the target patient population and their functional capability that can pose limitations to the use of some electronic devices or require special adaptations. Electronic devices present the big advantage of real-time data capture, which allows for monitoring on an ongoing basis via a dedicated web portal. This can increase patient compliance and help prevent or prompt identification of any issues that may impact data quality or patient safety.37 Even if use of PRO in clinical trials is encouraged, this should not represent an excessive burden for the patient with consequent impact on compliance and quality. Therefore, the number of PRO, their relevance for the specific trial, time needed for completion, and frequency of assessment should all be considered to find the right balance between study needs and patient comfort and acceptance.
Lastly, patients and site staff both need to be trained on PRO completion and delivery.
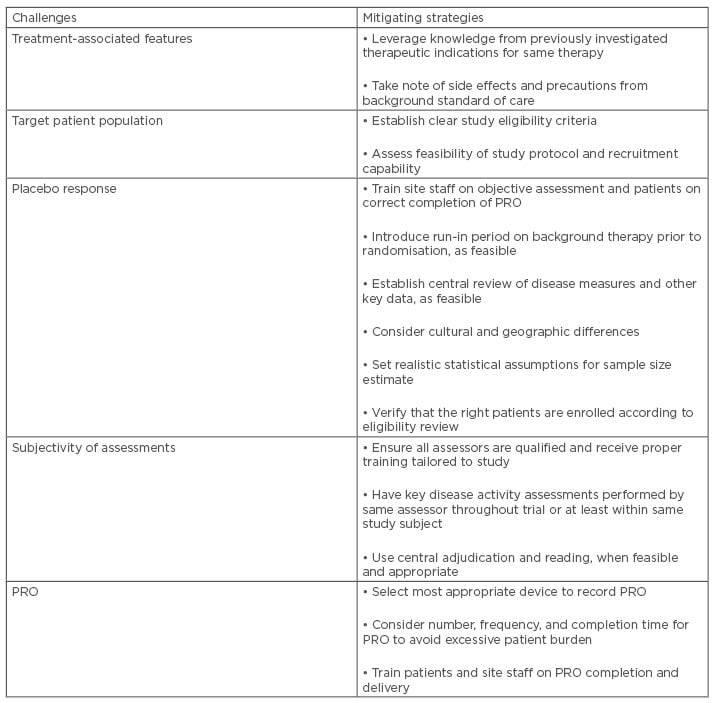
Table 2: Addressing challenges of chronic inflammatory disease trials.
PRO: patient-reported outcomes.
CONCLUSION
CID present several common features in terms of patient and treatment characteristics, and with respect to operational aspects of clinical trials. They also share important challenges that could be overcome or mitigated by adopting proper strategies, as summarised in Table 2. This supports an approach to CID as a largely interconnected therapeutic area where multidisciplinarity and application of common strategies and lessons learnt across different indications represent crucial factors for planning and executing clinical trials in an effective and timely fashion.








