Abstract
Introduction: Rheumatoid arthritis (RA) is associated with changes in skeletal health, including increased risk of fracture. This study used a novel technique, high-resolution quantitative CT (HRpQCT), to assess bone microarchitecture in patients with RA.
Methods: There were 59 patients (female: 41; male: 18) with RA recruited. They underwent dual energy X-ray absorptiometry and HRpQCT of the radius and tibia. The questionnaire information included age, sex, BMI, disease duration, comorbidities, medication use, smoking and alcohol consumption, rheumatoid factor (RF) or cyclic citrullinated peptide (CCP) status, and disease activity. HRpQCT results were compared with published estimated age and sex-specific values.
Results: There were 55 patients (female: 39; male: 16) who had either radial or tibial scans available. The mean age was 55.8 (standard deviation [SD]: 12.6) years and median disease duration was 11.4 years (interquartile range [IQR]: 6.3–19.4). Mean BMI was 27.2 (SD: 5.8). Forty-nine (90.7%) participants were RF or CCP positive, with disease severity ranked as severe in 33 (61.1%) patients and moderate in 20 (37.0%). Fifteen participants (27.8%) had previously taken steroids and 47 (85.5%) were receiving tumour necrosis factor inhibitor (TNF-i) medication. Radial trabecular number and density were lower than expected, and trabecular separation was greater than expected (p<0.05), though tibial results were similar (p<0.10 for trabecular number and separation). No difference in cortical values reached statistical significance in this sample. Previous use of steroids was associated with greater radial periosteal circumference (p<0.05, adjusted for sex) and use of TNF-i agents was associated with lower radial total and trabecular area (p<0.05, adjusted for sex).
Conclusion: Trabecular bone microarchitecture differences were observed among patients with RA. Further studies with larger numbers of participants are needed.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic, systemic inflammatory arthritis1-3 characterised by synovial inflammation and hyperplasia,3,4 autoantibody production,2-4 and destruction of cartilage and bone.2,3,5,6 It is relatively common, with an estimated prevalence of 0.5–1.0% in the population, and often affects the small joints of the hands and feet.4 The disease is known to cause bone erosions2,3,6 and periarticular osteopenia.1,6 A common and important comorbidity is a generalised reduction in bone mineral density (BMD),2,3,5-8 with patients with RA having an estimated two-fold increase in the frequency of osteoporosis compared to healthy controls.5,8 Multiple studies using dual energy X-ray absorptiometry (DXA) have demonstrated reductions in BMD in patients with RA at sites including the hip8-12 and the lumbar spine.10-12 This loss of bone is thought to occur early in the disease5,10,12,13 and is associated with higher disease activity,10,12,13 increased functional disability,8-11,13 and longer disease duration.10,11,13 Additionally, RA is associated with an increased risk of skeletal fractures of the hip14-17 and the vertebrae.16,18,19
Corticosteroids have been commonly used in patients with RA to control inflammation with great clinical efficacy,20 but they are also known to have negative effects on bones by increasing osteoblast apoptosis and osteoclast activity.21,22 It was traditionally thought that the reduced BMD and increased fracture risk observed in patients with RA were due in large part to steroid use in this cohort. However, whilst there is evidence that steroids do reduce BMD7-10,21,23,24 and increase fracture risk in RA,15-17,21 there is also evidence that their use has only a minimal effect on BMD.11,19,22 Additionally, with the development of biologic therapies, steroids are used less frequently and for shorter durations in patients with RA.20 One might speculate that bone health would be better in cohorts of RA treated with current therapeutic agents because of their strong anti-inflammatory effects and good clinical efficacy. However, a recent study that utilised the UK Biobank to evaluate this found that a diagnosis of RA remains associated with poorer bone health, as assessed by heel ultrasound, and an increased frequency of falls and fractures.24 It was also found that corticosteroids and conventional disease-modifying therapy, but not biologic therapy, were associated with lower epithelial BMD.24
Bone strength is not purely determined by BMD19,25 but also by bone quality, which is affected by bone remodelling, microarchitecture, and mineralisation of the bone matrix,25,26 and may contribute to risk of fracture.25 There is growing evidence that RA may increase skeletal fracture risk by impairing bone quality in a manner independent from BMD,5,19,24 potentially by altering the bone remodelling process.26 High-resolution peripheral quantitative CT (HRpQCT) is a useful tool to assess bone microarchitecture and can separate cortical and trabecular bone at distal sites, and give true measurement of compartmental volumetric bone density.7,26,27 Its use in the diagnosis or assessment of erosive disease progression in inflammatory arthritis has been well studied,26 but far fewer data are available that consider its utility in the assessment of generalised skeletal health. Three previous studies that have used this technology for this purpose have all focussed on the radius.28-30 They reported that both trabecular and cortical radial bone were severely affected in male and female patients with RA, with volumetric BMD in both compartments being reduced. In this study, the authors set out to study bone health in a group of patients with RA recruited from general rheumatology clinics, and specifically to extend sites of interest to include the tibia, which can also be assessed by HRpQCT. The following variables derived by HRpQCT at the radius and tibia in patients with RA were considered: bone area; trabecular number, density, separation, and thickness; and cortical density and thickness.
MATERIALS AND METHODS
Fifty-nine patients with RA were recruited to the study at the Osteoporosis Centre in Southampton, UK. Participants were approached in general rheumatology clinics, with many contacted through the Southampton Biologics service. They completed a self-reported questionnaire on age, sex, height, weight, time since RA diagnosis, comorbidities, medication use (including tumour necrosis factor inhibitors [TNF-i] and steroids), and smoking and alcohol consumption. Rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP) status was recorded, as well as the physician and patient assessment of disease activity from patient records.
Participants underwent DXA of the total hip and lumbar spine using a Hologic Discovery™ machine (Hologic Inc, Bedford, Massachusetts, USA). HRpQCT scans of the distal radius and tibia were obtained using XtremeCT-I, (Scanco Medical, Bassersdorf, Switzerland). A stack of 110 parallel HRpQCT slices were acquired with an isotropic voxel size of 82 µm. The standard evaluation and cortical porosity scripts were run to obtain estimates of various indices of bone health, including cortical and trabecular BMD and trabecular thickness. Fifty-six of the participants had radial scans, of which 25 were excluded because of excessive motion artefact (Grade 4 or 5 on the standard grade system), leaving 31 patients with useable radial scans available. Fifty-seven of the participants had tibial scans, of which four were excluded because of excessive motion artefact, leaving 53 patients with useable tibial scans available. In total, 55 participants had either radial or tibial scans available. Results were compared with estimated age and sex-specific values calculated from the formulae in the paper by Dalzell et al.31 Ethical approval was obtained from Hertfordshire REC (reference 13/EE/0215).
STATISTICAL ANALYSIS
Descriptive statistics for continuous variables were expressed as mean and standard deviation (SD) or median and interquartile range (IQR). Categorical variables were expressed as frequency (N) and percentage. Wilcoxon signed-ranks tests were used to compare the HRpQCT values with estimated age and sex-specific values calculated from the formulae in the paper by Dalzell et al.31 The HRpQCT outcomes were transformed to Fisher–Yates z-scores using the Fisher–Yates rank-based normal transformation to normalise the data. Linear regression analyses were used to examine the associations between patient characteristics and the HRpQCT outcomes (as z-scores) after adjusting for sex. Analyses were performed using Stata version 14 (StataCorp, College Station, Texas, USA).
RESULTS
The characteristics of the study population are shown in Table 1. The mean age of the 55 participants was 55.8 (SD: 12.6) with 39 participants (70.9%) being female. Median disease duration was 11.4 years (IQR: 6.3–19.4). Mean BMI was 27.2 (SD: 5.8). Forty-nine (90.7%) participants were RF or CCP positive, with disease severity ranked as severe in 33 (61.1%) patients and moderate in 20 (37.0%). Fifteen participants (27.8%) had previously taken steroids and 47 patients (85.5%) had previously taken TNF-i. The median (IQR) T and Z scores at the lumbar spine were -0.8 (-1.8, 0.3) and -0.1 (-0.8, 1.3) respectively; corresponding figures at the total hip were -0.8 (-1.4, 0.2) and -0.2 (-0.7, -0.6), respectively. Three patients were taking anti-osteoporosis medication at the time of scanning; adjusting for taking this medication did not affect the results. The characteristics of the 59 participants were considered, comparing those with (n=31) and those without (n=28) useable radial scans; there were no statistically significant differences between them. A similar comparison of those with (n=53) and without (n=6) useable tibial scans again showed no statistically significant differences.
Usable radial scans were obtained from 31 of the 55 recruited patients. Expected values were calculated from age and sex using published means.31 The p-values were calculated by comparing the actual values with the expected values using Wilcoxon signed-ranks tests. Compared to expected values, patients with RA had significantly lower trabecular number (median: 1.82 mm-1 versus 1.93 mm-1, respectively; p=0.014) and significantly lower trabecular density (median: 140 mg/cm3 versus 148 mg/cm3, respectively; p=0.048) (Table 2). They also had significantly lower trabecular separation (median: 0.486 versus 0.488, respectively; p=0.046). Steroid-use was associated with greater radial periosteal circumference (regression coefficient: 0.561 z-score; p=0.03) and use of biologic agents was associated with lower radial total area (regression coefficient: -0.585 z-score; p=0.02) and lower trabecular area (regression coefficient: -0.581 z-score; p=0.03), after adjustment for sex.
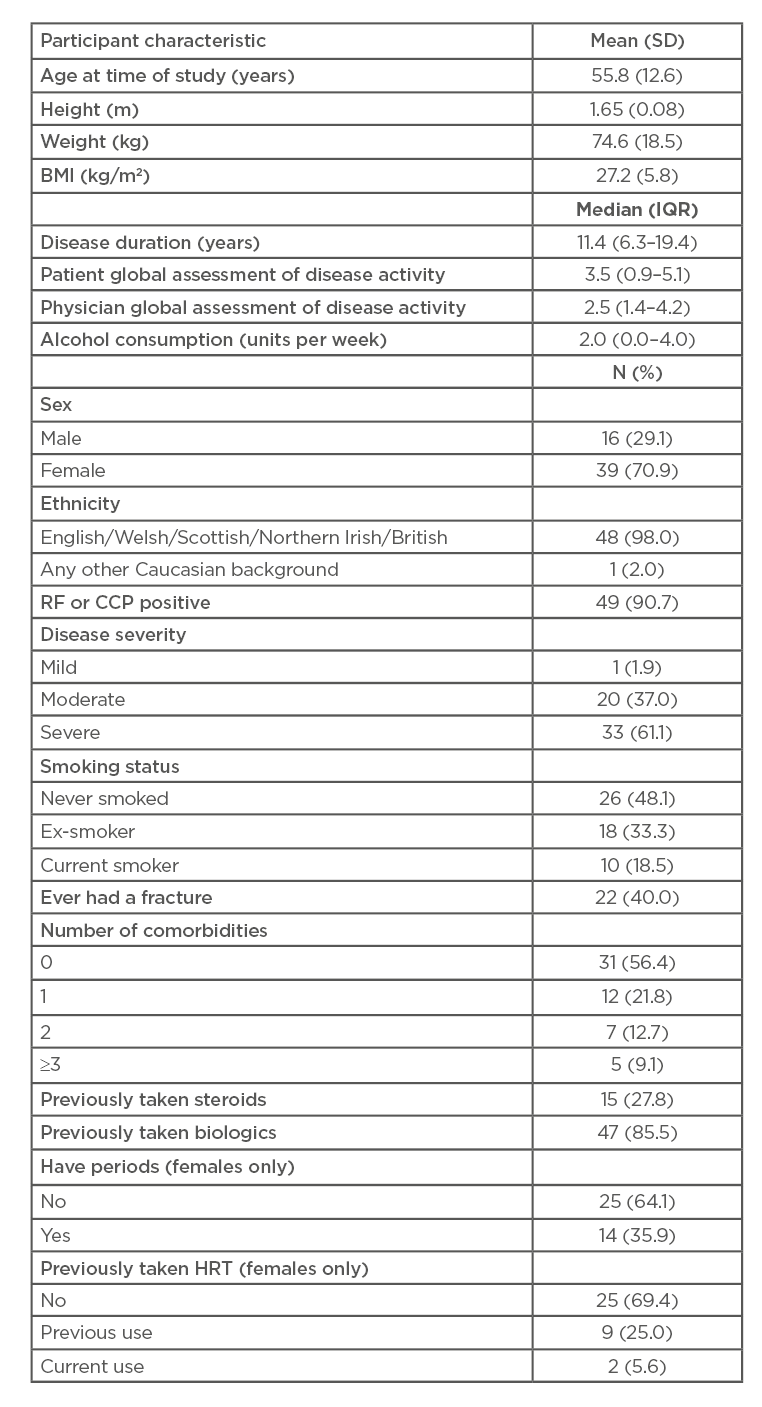
Table 1: Participants’ characteristics.
CCP: cyclic citrullinated peptide; HRT: hormone replacement therapy; IQR: interquartile range; RF: rheumatoid factor; SD: standard deviation.
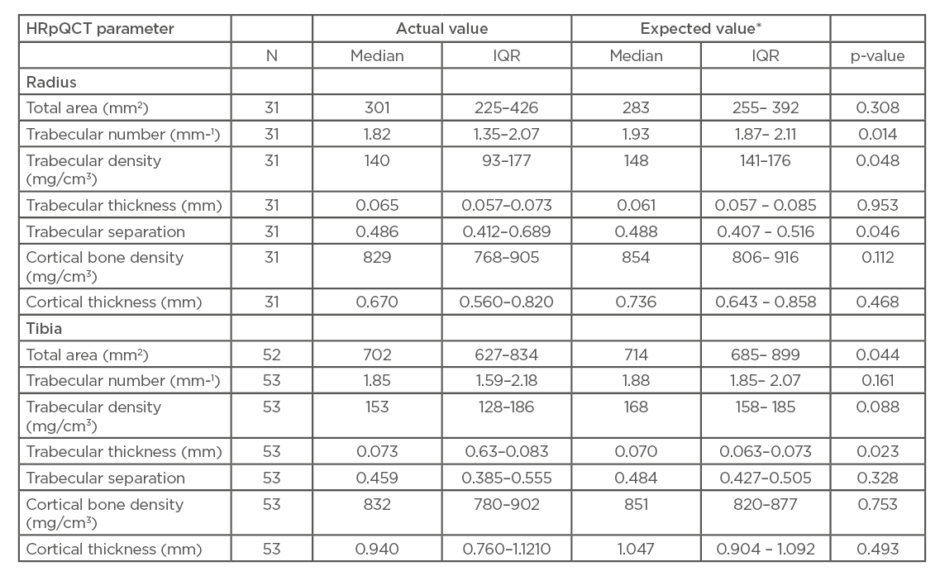
Table 2: Comparison of mean high-resolution quantitative CT (HRpQCT) parameters from participants with expected values.
HRpQCT: high-resolution quantitative computed tomography; IQR: interquartile range.
*Expected values are the age- and sex-specific values calculated from the formulae in the paper by Dalzell et al.31
Radial cortical bone density and cortical thickness were reduced in patients with RA relative to expected values, but differences did not reach significance.
Figure 1 shows a typical tibial scan. Usable tibial scans were obtained from 53 of the 55 recruited patients. Tibial total area was significantly decreased in patients with RA compared to expected values (median: 702 mm2 versus 714 mm2, respectively; p=0.044) and tibial trabecular thickness was significantly increased (median: 0.073 mm versus 0.070 mm, respectively; p=0.023). While similar trends towards reduced volumetric BMD in cortical and trabecular compartments were seen at the tibia, and trabecular separation was lower, these effects were less noticeable than at the radius and failed to reach statistical significance. No association between tibial indices and previously taking steroids or biologics could be found in this study population.
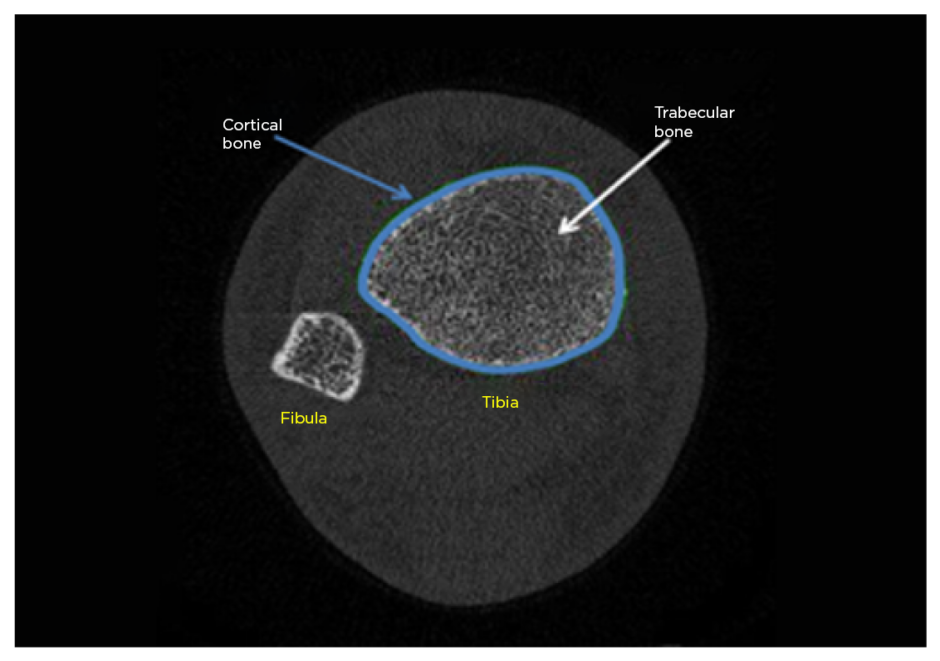
Figure 1: Typical tibial high-resolution quantitative CT (HRpQCT) scan.
DISCUSSION
The results have shown significant differences in trabecular bone parameters between patients with RA and healthy controls that were more pronounced at the radius. These results are in line with previous studies using HRpQCT, which have also shown reduced radial trabecular density,28-30 reduced trabecular number,30 and increased trabecular network inhomogeneity28-30 in patients with RA, relative to controls. Reductions in trabecular number have been shown to be related to disease duration,30 indicating ongoing loss of trabecular bone over time. There is evidence from previous HRpQCT studies that healthy patients who subsequently present with fractures have lower radial trabecular density, number, and thickness,32,33 and higher trabecular separation32 compared to nonfractured age-matched controls, suggesting an important contribution of bone microarchitecture to fracture risk.
As patients with RA in this cohort demonstrated similar alterations in their radial microarchitecture, it is possible that these alterations in bone quality could contribute to the increased fracture risk seen in this study. Few studies have considered whether wrist fracture is particularly increased in patients with RA, but despite an increased risk relative to controls, other sites (e.g., hip, pelvis, humerus) seem more vulnerable.17 Whilst there is evidence that both trabecular and bone are deficient in patients with RA,28-30 any differences in cortical microarchitecture did not reach significance in this patient population.
The bone loss that occurs in RA is known to be related to inflammation.5,6 T cells stimulate the production of inflammatory cytokines such as TNF-α and IL-1 from monocytes, macrophages, and synovial fibroblasts,2-4 which in turn have the ability to induce expression and release of the cytokine receptor activator of NF-κB ligand (RANKL).1,2,4 RANKL binds to its receptor RANK and mediates bone resorption by osteoclasts.1,2,4,5 Additionally, IL-1 and TNF-α are able to directly upregulate osteoclast bone-resorbing capacity and stimulate the development of osteoclast precursor cells into mature osteoclasts.1,4 Several studies have shown patients with RA to have increased bone resorption markers compared to normal controls,7,34 which suggests they develop an imbalance between bone formation and resorption,1 leading to an overall loss of bone. Zhu et al.29 found a correlation between volumetric density; microstructure indices; and disease activity, severity, and levels of inflammatory cytokines, suggesting that inflammation may contribute to the observed impairments in bone quality as well as reducing BMD.
Whilst corticosteroids have been shown to reduce BMD7-10,21,23,24 and to increase fracture risk in RA,7,15,21,24 their anti-inflammatory properties have a beneficial effect on bone which may outweigh the negative effects.22 This leads to an overall improvement of BMD in patients with RA treated with steroids,6,10,11,23,35 particularly in those who have severe disease with high levels of inflammation. Some studies have identified that RA itself can reduce BMD9 or increase the risk of fracture in a manner that is partially independent from the increased risk incurred by steroid use.6,15,16 Additionally, there is evidence that the bone loss and increased fracture risk seen with steroids is reversible after discontinuation of therapy.21 Current guidelines suggest that steroids still have a place in RA management as an adjunct to conventional disease-modifying therapies, but that their use should be limited to the lowest possible dose for the shortest possible duration when treating disease flares or switching from one disease-modifying drug to another.20 In this study population, only 15 patients (27.8%) had ever taken steroids, therefore it was not possible to comment on any potential effect they may have had on bone microarchitecture. There is evidence that steroid use causes reduced cortical and trabecular BMD21,36 and that high doses may cause cortical thinning,30 but other studies have shown that exposure to steroids does not significantly affect bone density or microstructure.28,29 The finding of increased periosteal circumference in patients who have received steroids as part of their disease management could reflect an ‘aging phenotype’ of bone, represented by an enlarged periosteal circumference to compensate for endosteal bone loss. This would be interesting to study in larger samples of patients who have received these drugs.
The efficacy of biologic therapies such as TNF-i and anti-IL-1 drugs supports the pathogenic role of these cytokines in the disease process,1,4 but the effect of such treatment on bone health remains uncertain. In this study population, most patients (85.5%) had taken TNF-i. Based on the knowledge that inflammation stimulates bone resorption, it is reasonable to hypothesise that tight control of inflammation with medication should reduce bone damage in RA patients.2,25 Improvement in BMD37 or stabilisation and reduction of bone loss has been recorded with biologic treatments that target specific cytokines, but results have been inconsistent.36-41 The effect of such treatments is thought to be more pronounced on generalised bone loss rather than local damage, as evidenced by the continuing hand bone loss seen in several studies.38,41 However, some biologic therapies have been shown to reduce the progression of bone erosions.36 There is also evidence that anti-TNF treatment may suppress bone resorption in RA.37 Kim et al.42 showed that biologic therapy does appear to reduce fracture risk compared to methotrexate or other nonbiological disease-modifying drugs, but further studies on fracture rate post-initiation of biologic therapies are needed.36 In the UK Biobank data there was no statistically significant association between biologic therapy and epithelial BMD, falls, or fracture for participants with RA.24 Of note, in a longitudinal study by Guler–Yuksel et al.,35 no difference in BMD loss was observed between patients managed with four different treatment strategies (including corticosteroids and anti-TNF medications). Additionally, in a study by Kocijan et al.,30 no specific associations between biological agents and bone microarchitecture could be identified. Both of these factors suggest that the direct impact of a specific treatment modality on bone strength may be less important than its effectiveness at suppressing inflammation and reducing the related damage to bone.30,35 In this study a high proportion of patients recruited were receiving biologic therapies, limiting the opportunity to study relationships between disease activity and bone health. However, despite this, and even in this relatively small sample size, trabecular alteration in the bones of patients with RA was observed.
Another mechanism that might lead to an association between RA and poor bone health is lack of weight-bearing physical activity because of the active disease.6 In this population, patients with RA had increased tibial trabecular thickness compared to normal controls, which was an unexpected finding. That there was a less apparent effect of RA at the tibia, the weight-bearing site of interest, or as assessed by total hip BMD, suggests this factor may be less contributory than systemic inflammation. In a study by Boutroy et al.,27 women with osteoporosis had reduced trabecular number and thickness and increased trabecular separation compared to women with osteopenia, which is similar to the radial findings from patients with RA in this study. Additionally, women with osteopenia and fractures had reduced radial trabecular density and more heterogeneous trabecular distribution than nonfractured women with osteopenia,27 again suggesting this cohort may have impairments in their bone quality which put them at increased risk of fracture. Previous studies have shown a link between trabecular parameters of the radius and skeletal fracture that is relatively independent of DXA-measured bone density,32 highlighting the value of HRpQCT for assessing bone parameters where it is available, especially as forearm BMD is rarely performed in clinical practice. As the majority of fractures in postmenopausal women occur in those who have an osteopenia rather than an osteoporotic range of BMD,43 it is highly likely that other factors contribute to fracture risk.27 As patients with RA may have impairments in their bone quality that are independent from BMD,19,25,26 there are recommendations that there should be a lower threshold for starting anti-osteoporotic treatment in this cohort to protect them from fracture.25 Additionally, patients with RA may be at increased risk of falling because of pain, functional impairment, and physical disability,24 which again may impact on their fracture risk independently of bone quality.7,44
Despite advances in biologic therapies and the treatment of RA, there is still evidence that these patients are still at increased risk of fracture compared to healthy controls.17,45 This could be because of the fact that even optimum treatment does not completely suppress inflammation in RA,2 or because of alterations in bone microarchitecture that occur independently from BMD changes as a result of the disease. There is also evidence that the rates of skeletal fracture in patients with RA are increasing despite new developments in treatment, which may be because of the aging population.46 All of these factors further highlight the importance of being aware of bone health in patients with RA and initiating therapies to protect against skeletal fracture.25 There is evidence from several studies that low proportions of patients with RA who meet the criteria for anti-osteoporotic therapy are actually taking the medication,23,35 and poor adherence to such therapies is well recognised.25
There are, of course, many limitations to this pragmatic study and more work in larger patient populations is very important, particularly to include patients with early RA, and those with very well controlled disease. The population studied was relatively small and the authors did not use their own controls, but compared the results from the cohort with the expected values from a different UK population. A validated tool, the physician global scale,47 was used to assess disease activity. However, other recognised scores such as the Disease Activity Score-28 (DAS28) were not available in this dataset. Additionally, many of the patient characteristics were self-reported, and may have been subject to bias. Steroid and biologic use were considered as binary variables and therefore the effect of dose or length of usage could not be analysed. However, the results serve to highlight the skeletal effects of RA and encourage consideration of volumetric BMD in studies where HRpQCT are being undertaken. They also emphasise the importance of recognising bone quality alongside BMD when considering skeletal health in this population.
CONCLUSION
This study adds to the evidence that patients with RA have alterations in their radial trabecular bone microarchitecture compared to healthy controls, which may contribute to the increased fracture risk seen in this population. Clinicians should be aware of the direct effect of RA on bone microarchitecture, even in patients who are optimally treated using biologic therapies, and should consider implementing strategies to protect bone, such as the use of anti-resorptive medications. In an ageing population, the prevalence of osteoporosis and rate of fragility fractures in patients with RA is likely to increase, and there may be a limitation to the reduction in fracture risk that can be achieved through improving BMD alone. Further studies in larger study samples are indicated, as are studies looking at skeletal fracture as the endpoint.







