Abstract
Axial spondyloarthritis (axSpA) is a chronic inflammatory condition, with an age of onset almost exclusively under 45 years. Although symptoms are initially centred on the sacroiliac joints and spine, extraspinal manifestations are common and add considerably to the burden of disease. In this narrative review, the authors provide an update on the epidemiology of the disease and briefly summarise the pathophysiology. The authors detail the clinical manifestations of axSpA, including an overview of axial features, peripheral manifestations, and associated comorbidities. The authors outline the current outcome measures used in the assessment of patients. Finally, the authors provide a summary of the general principles of treatment and briefly outline the role of patient education in the management of individuals with axSpA.
INTRODUCTION
Axial spondyloarthritis (axSpA) is a chronic inflammatory condition that predominantly affects the sacroiliac joints and spine. It is a type of spondyloarthritis (SpA) that refers to a group of inter-related conditions including psoriatic arthritis (PsA), reactive arthritis, and arthritis associated with inflammatory bowel disease (IBD).1 Ankylosing spondylitis (AS) was the prototype SpA condition, diagnosed when characteristic changes of sacroiliitis are seen on an X-ray of the pelvis in conjunction with clinical criteria.2 It has been increasingly recognised that the characteristic radiographic changes of AS could take many years to develop, thus excluding a large group of people with suggestive symptoms, but normal X-rays. This led to the development of the Assessment of SpondyloArthritis international Society (ASAS) criteria in 2009, which recognised a radiographic stage broadly similar to AS, as well as a non-radiographic stage (nr-axSpA).3 This allowed individuals with earlier or less severe disease, who had suggestive clinical features and sacroiliitis on magnetic resonance imaging, to be classified as axSpA.
EPIDEMIOLOGY
AxSpA typically occurs in the third decade of life, and almost exclusively before 45 years of age. Historically, AS was considered to be a male-dominated disease, with early literature reporting ratios of up to 10:1. However, more recent estimates put that ratio at closer to 3:1, with virtually no difference seen in the distribution of nr-axSpA between men and women.4
The major histocompatibility complex 1 human leukocyte antigen (HLA)-B27 allele is strongly associated with axSpA, and is found in 74–89% of affected individuals.5 The prevalence of axSpA is typically greater in populations with a higher background prevalence of HLA-B27.4 In Europe, the prevalence of HLA-B27 varies from 2% to 25% and is highest in Scandinavian countries.6 In contrast, HLA-B27 is rare in Japan and Arab countries, and almost non-existent in some populations such as indigenous tribes of South America.6 The Pawaia tribe in Papua New Guinea has the highest prevalence of HLA-B27 worldwide, with 53% of people affected.7
In 2016, Stolwijk et al.8 systematically estimated the global prevalence of axSpA between 0.36% and 0.70% and AS between 0.20% and 0.25%. The lowest prevalence was in Southeast Asia with a pooled prevalence of 0.2% and the highest in the northern Arctic communities. The native Eskimo (Inuit) population demonstrated a strikingly high prevalence of SpA of 2.5%, with the prevalence of HLA-B27 also high, affecting up to 40% of the study population.9
However, in reality it is difficult to know the true prevalence of axSpA because of a lack of population studies and a continued under-recognition and underdiagnosis of axSpA. In addition, delay to diagnosis has been a longstanding challenge in the management of axSpA, with mean delays of approximately 7 years reported in a large meta-analysis of 23,883 individuals.10 Long delays to diagnosis are associated with a number of worse outcomes in axSpA, such as more active disease, greater chance of disability, and increased healthcare costs.11 The delay to diagnosis is more common in women than men.10 Women are less likely to report typical inflammatory back-pain symptoms, and more likely to report widespread pain; misdiagnoses such as fibromyalgia (FM) are more commonly made in the female population,12 which may also exacerbate diagnostic delay. A negative HLA-B27 also appears to be associated with diagnostic delay.13 If undiagnosed or under-treated, axSpA may lead to continuous pain, stiffness, and fatigue, and may ultimately lead to a reduction in quality of life (QoL).
PATHOPHYSIOLOGY
Most pathogenesis studies to date have focused on AS rather than axSpA. It appears to develop through complex interactions between genetic background and environmental factors. Studies performed on monozygotic twins and familial aggregation studies suggest that AS has a heritability of above 90%.14,15 HLA-B27 positivity is also strongly linked with AS, occurring in only about 5% of the general population, but more than 90% of individuals with AS.16 Exactly how HLA-B27 predisposes to AS is not yet fully understood, but several hypotheses have been put forward:17
- Arthritogenic peptide hypothesis: the specific sequence of amino acids found in the peptide-binding groove of HLA-B27 might bind a peptide which elicits a cytotoxic T-cell response cross-reactive with a B27/self-peptide combination, i.e., molecular mimicry.16
- HLA-B27 misfolding hypothesis: it is thought that the specific sequence of amino acids in the peptide-binding groove causes a propensity for HLA-B27 to misfold in the endoplasmic reticulum, leading to a pro-inflammatory stress response; however, evidence to date is largely limited to rat studies.18
- Cell-surface B27 free heavy chain expression and immune recognition hypothesis: HLA-B27 tends to form homodimers, which bind to free heavy chains expressed on the cell surface, thus triggering a pro-inflammatory process.16
However, despite this strong association, HLA-B27 contributes only 33% of the total heritability of AS.16 Genome-wide association studies have additionally detected several genes associated with AS.19 Barrier damage to the skin or gut surfaces may also be relevant to pathogenesis.20 Microbial infection appears to act as a triggering factor.21
CLINICAL FEATURES
Axial Symptoms
Patients typically present with inflammatory back pain (IBP), characterised by an insidious onset of lower back and alternating buttock pain that worsens with inactivity and improves with exercise and non-steroidal anti-inflammatory drugs (NSAID). Patients may report waking at night with lower back pain, particularly in the latter stages of sleep. IBP is also associated with morning stiffness that usually lasts more than 30 minutes.22 Determining whether lower back pain is inflammatory or mechanical can be challenging because lower back pain is very common in the general population, with 38% experiencing lower back pain for at least 1 day every year.23 However, of all those with chronic lower back pain, IBP represents only approximately 5%.24 Many attempts have been made to classify IBP (Table 1),22,25,26 with the following features considered important in differentiating between IBP and other common causes of back pain:22
- Age: onset of axSpA after the age of 45 years is exceedingly rare.
- Duration of pain: non-IBP is often self-limiting.
- Onset of pain: non-IBP is often acute in onset.
- Diurnal variation: pain and stiffness in axSpA-related IBP tends to be worse in the second half of the night and early morning.
- Response to exercise: IBP responds well to exercise, a feature characteristic of many inflammatory conditions.
- Location: alternating buttock pain can indicate inflammation of sacroiliac joints.22,26
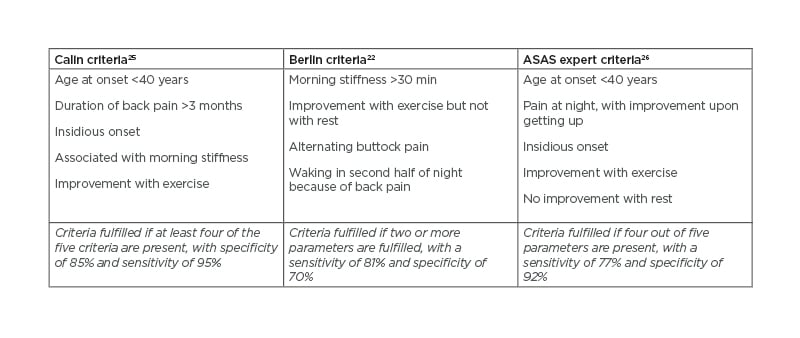
Table 1: Three separate criteria for inflammatory back pain.
ASAS: Assessment of SpondyloArthritis international Society.
Italics refer to respective criteria fulfilment.
Peripheral Disease
Peripheral arthritis can affect up to half of individuals with axSpA, has a higher prevalence in the Latin American population compared to other geographic regions, tends to be more common in the lower limbs, and is typically oligoarticular.27 Peripheral arthritis in axSpA may affect any joint, but is typically asymmetric. It is important to recognise peripheral arthritis, as its presence may direct management. Conventional synthetic disease-modifying antirheumatic drugs have a role in managing peripheral arthritis, whereas they are ineffective for axial disease.
Entheses are the sites of attachment of tendons or ligaments to bone, and enthesitis (inflammation of entheses) occurs in 44% of individuals with axSpA, with a predominance for lower limbs.27 The heel (Achilles tendon or plantar fascia) is the most common site of enthesitis in axSpA, with most affected individuals having an intermittent course.27 It can be difficult clinically to differentiate between enthesitis and arthritis, due to the anatomic overlap between entheses and joints.28
Dactylitis or ‘sausage digit’ involves inflammation of an entire digit (either a finger or toe), and is caused by flexor tenosynovitis, in combination with soft-tissue oedema.29 Dactylitis can occur in up to 8% of individuals with axSpA,27 although it is more commonly seen in PsA.30 Interestingly, a meta-analysis explored the difference in the prevalence of peripheral manifestations between patients with AS and nr-axSpA and found no significant differences between the two groups.31
Extra-Articular Manifestations
Acute anterior uveitis (AAU), IBD, and psoriasis (PsO) are three conditions that are over-represented in axSpA and are thus considered extra-articular manifestations of the disease.
AAU is the most common extra-articular feature of axSpA, with a reported prevalence of 26–33%.32 AAU associated with axSpA typically presents acutely, often with a 1- to 2-day prodrome, and tends to be unilateral, with circumlimbal hyperaemia, pain, photophobia, and visual impairment, with subsequent attacks often affecting the other eye. AAU more commonly occurs in a HLA-B27-positive patient cohort.33 Visual prognosis in AAU associated with axSpA is excellent, with most individuals regaining full vision within 2 months.34
PsO is another notable feature of axSpA, affecting approximately 10% of individuals.32 This disease feature must be distinguished from a diagnosis of PsA, which may present similarly. Patients with axSpA tend to have more back pain at presentation and score higher on physician score global indices when compared to PsA.35 Interestingly, the presence of PsO in axSpA is an independent risk factor for increased entheseal damage.36
Clinically evident IBD is noted in 6–14% of AS patients,32 with Crohn’s disease occurring more commonly than ulcerative colitis. However, microscopic evidence of IBD was noted in up to 60% of AS patients, suggesting a significant proportion of clinically silent disease.37 Faecal calprotectin is a non-specific marker for gut inflammation. In a 5-year longitudinal study, higher faecal calprotectin levels at baseline were associated with the development of Crohn’s disease, as well as more severe AS disease clinically, but without any relationship to gut symptoms, suggesting that inflammation in the gastrointestinal tract and musculoskeletal system are linked.38
COMORBIDITY IN AXIAL SPONDYLOARTHRITIS
Mortality in axSpA is increased when compared with age- and sex-matched controls.39,40 Some of this excess mortality can be explained as a direct consequence of the disease, such as death because of neurological deficits from vertebral fractures.41 However, comorbid conditions, in particular cardiovascular disease, are shown to be a leading cause of death in axSpA.39,40
A comorbid condition can be defined as any distinct additional clinical entity that has existed or that may occur during the clinical course of an individual who has the index disease under study.
The ASAS-COMOSPA study was a large, multinational, cross-sectional study that outlined the profile of comorbidities occurring in individuals with SpA.42 The most prevalent comorbidity was osteoporosis, affecting 13% of the cohort. Diagnosis of osteoporosis in axSpA using dual-energy X-ray absorptiometry may be difficult in advanced structural disease because of the presence of osteoproliferation, which can falsely increase bone mineral density. Lateral views of the spine are a promising technique for overcoming this.43
Almost 4% of the ASAS-COMOSPA cohort had cardiovascular morbidity,42 with a lower prevalence in axSpA individuals compared with those with peripheral SpA.44 Hypertension and smoking have been shown to be more pervasive in an axSpA population.45
Depression is common in axSpA, with Zhao et al. reporting a prevalence of 15% showing at least moderate depression in a systematic review.46 Obesity is another comorbidity that is shown to be common in axSpA, affecting 15–27% of axSpA cohorts.47,48 Of note, patients with concomitant obesity tend to be less responsive to conventional therapy in axSpA.49
Comorbidities in axSpA are of clinical relevance, as they have been shown to add to the burden of disease. Increasing comorbidity burden is associated with more active disease and worse spinal mobility.47 The presence of comorbidities in SpA adversely affects physical function, work ability, QoL, and increases healthcare expenditure.47,50 Despite this known burden of comorbidities in axSpA, optimal screening for them is poor, with the ASAS-COMOSPA study demonstrating that only half of participants had an adequate assessment of their cardiovascular status, and optimal cancer screening was only performed in 11–44% of participants.42 Less than 20% of individuals with axSpA have an objective assessment of their bone mineral density.47 Treatment of comorbidities is also suboptimal, with less than one-quarter of a Dutch cohort of patients with AS treated for hypertension or hypercholesterolaemia achieving treatment targets.45
FM is a non-inflammatory comorbidity characterised by widespread pain and fatigue, with an estimated one-in-six prevalence in axSpA.51 Patients who met the criteria for FM reported significantly worse disease activity and function, as well as a lower QoL.52 In patient-reported outcomes (PRO), low disease activity or disease remission is less likely to be achieved in patients with FM.53 The presence of FM appears to have a negative impact on response to biologics,54 with shorter retention times.55 For this reason, it is important to screen patients with AxSpA for FM because it may affect patient outcomes.
OUTCOME MEASURES
AxSpA is a complex multifaceted condition, and assessment of outcomes must reflect this. A number of validated outcome measures have been developed in axSpA, which deliver a comprehensive overview, subjectively and objectively, of many different domains of disease. Here, the authors collate scoring systems in axSpA that detail disease activity, patient function, QoL, radiological assessment, as well as objective measurement of enthesitis and dactylitis.
Bath Ankylosing Spondylitis Disease Activity Index
The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) is a validated PRO that measures fatigue, spinal pain, peripheral joints, enthesopathy, and early-morning stiffness severity and duration on a scale of 0–10.56 Scores of 4 or greater suggest active disease, which may require alteration of therapy.57 The benefit of the BASDAI is that it is a simple, patient-focused assessment assessing a wide variety of axSpA features. One of its drawbacks is that objective evidence of inflammation such as C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR) is not assessed in the BASDAI. While the BASDAI did not initially include nr-axSpA patients, it has since been suggested that it may also be used in this patient cohort.58
Bath Ankylosing Spondylitis Functional Index
The Bath Ankylosing Spondylitis Functional Index (BASFI) is a patient-reported assessment for everyday tasks, also scored on a scale of 0–10, using either a numerical or a visual analogue.59 This scoring tool is validated, quick and easy to use, and sensitive to changes in the disease.60 Similar to the BASDAI, this tool had initially been validated for AS and not nr-axSpA.
Ankylosing Spondylitis Disease Activity Score
The Ankylosing Spondylitis Disease Activity Score (ASDAS) was developed to include objective measures of inflammation that were not present in the BASDAI. It is closely associated with the Disease Activity score utilised in rheumatoid arthritis. The ASDAS incorporates three questions from the BASDAI (back pain, peripheral pain, and early-morning stiffness), patient global assessment, and acute-phase reactant using either ESR or CRP.61,62 Disease activity is subsequently classified into inactive, moderate, high, and very high disease activity states.63 The ASDAS has demonstrated validity and is more sensitive to treatment than previous scoring metrics.64 A notable disadvantage of the ASDAS is CRP or ESR may not be available on clinic days. Also, a raised CRP or ESR may not always reflect disease activity.
Health Assessment Questionnaire
The Health Assessment Questionnaire (HAQ) is a PRO questionnaire, which initially focused on pain, disability, medication effects, costs, and mortality. Answers are assessed from 0–3, where 3 indicates more severe disability.65
Ankylosing Spondylitis Quality of Life
The Ankylosing Spondylitis Quality of Life (ASQoL) questionnaire is comprised of 18 questions that aim to accurately assess QoL in patients with AS.66 It may also be used in nr-axSpA. Each question is yes/no, with each ‘yes’ scoring one point. The points are summed, giving a total score between 0 and 18, with a higher score signifying a worse QoL. Difficulties with the AS QoL include failure of a yes/no answering system to fully encapsulate a patient’s sense of wellbeing.
Bath Ankylosing Spondylitis Metrology Index
The Bath Ankylosing Spondylitis Metrology Index (BASMI) differs from previous scoring systems in that it assesses objective clinical measurements rather than PRO. BASMI assesses spinal mobility on a scale of 0–10.67 The composite score measures cervical rotation, wall-to-tragus distance, lateral flexion, modified Schober’s test, and intermalleolar distance. Higher scores reflect more significant restriction. It is a quick and reproducible assessment that demonstrates high sensitivity.
Modified Stoke Ankylosing Spondylitis Spinal Score
The Modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) was developed to assess radiographic changes in AS.68 It uses lateral spinal X-rays to quantify radiological damage on the anterior aspect of the spine from the lower border of C2 to the upper border of T1, plus the lower border of T12, all five lumbar vertebrae, and upper sacrum. This scale scores each vertebra border on a 0–3 scale: 0 is normal; 1 shows sclerosis erosions or squaring; 2 is for obvious syndesmophyte formation; and 3 shows total bridging syndesmophyte formation. A summed score of 0 indicates a normal spine, and 72 a completely ankylosed, or ‘bamboo’, spine.
Maastricht Ankylosing Spondylitis Enthesitis Score
The Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) is a scoring system of enthesopathy in patients with spondyloarthritis. Thirteen sites are assessed for discomfort on clinical examination in binary terms of tender or non-tender.69 This scoring system is more user-friendly than previous enthesitis measurements such as the Mander Enthesis Index (MEI). High enthesitis scores correlate well with high disease activity scores.
Leeds Dactylitis Instrument
The Leeds Dactylitis Instrument (LDI) is a validated clinical measurement of dactylitis. Dactylitis was defined as an increase of 10% or more of the size of the contralateral unaffected digit.70 Measurement of degree of tenderness is also assessed clinically in order to complete the score. High scores are associated with worse dactylitis.
GENERAL TREATMENT OVERVIEW
Although a detailed overview of treatment is beyond the scope of this narrative review, the authors here outline the general principles of management of axSpA, based on existing recommendations(Figure 1).58,71 Briefly, the management of axSpA may be classified into non-pharmacological and pharmacological treatment, and treatment should be tailored according to the manifestations of the disease, the severity of symptoms, the clinical status of the patient, and the expectations and wishes of the patient.58 The importance of regular exercise and avoiding smoking must be emphasised to each patient, regardless of other treatment modalities. If the patient is symptomatic, regular NSAIDs are typically the first line of pharmacological treatment. If there is insufficient response with NSAIDs, patients with predominantly axial disease should be assessed for biologic disease-modifying anti-rheumatic drugs. Currently, anti-TNFs, anti-IL-17, and JAK inhibitors are licensed for axSpA treatment (Table 2).
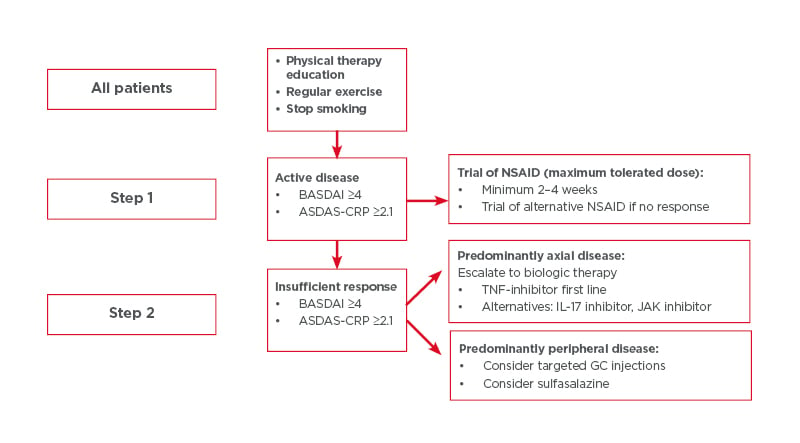
Figure 1: Steps for all patients taking part in physical therapy education, regular exercise, and who have stopped smoking.
ASDAS-CRP: Ankylosing Spondylitis Disease Activity Score–C-reactive protein; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; GC: glucocorticoid; NSAID: non-steroidal anti-inflammatory drug.
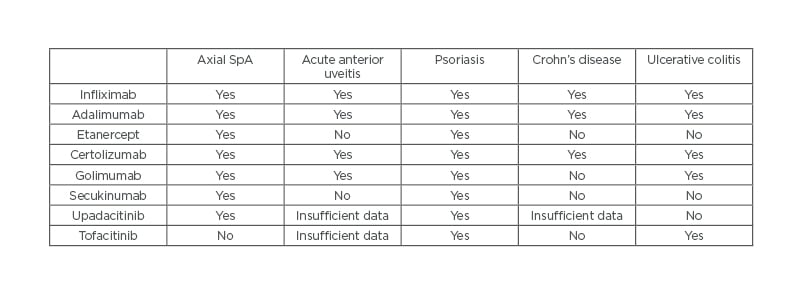
Table 2: Indications for biologics and JAK inhibitors.
SpA: spondyloarthritis.
THE ROLE OF THE PATIENT IN DISEASE MANAGEMENT
For too long, patients were often excluded from decisions regarding management of their condition. This has been addressed with the publication of the European League Against Rheumatism (EULAR) recommendations for patient education in inflammatory arthritis.72 Shared decision-making between the patient and rheumatologist is now an overarching principle in the recommended management of axSpA.58
To ensure that the patient can partake in all elements of this shared decision, education is key and refers to all activities that ensure an individual is well-informed about their condition, from education about treatment options to general health education and health promotion.72 Patient education, which can range from patient information leaflets to structured educational programmes, has been shown to increase adherence to treatment and promote patient self-management.73
The rheumatology nurse plays a key role in educating patients, with the updated 2018 EULAR guidelines recognising the importance of rheumatology-trained nurses in providing needs-based education to individuals affected with chronic inflammatory arthritis.74 The Educational Needs Assessment Tool (ENAT) is one instrument that can accurately assess an individual’s educational need and has been validated in many rheumatic conditions, including AS.75 Using the results of the ENAT to guide the education of a patient can lead to improved outcomes.76 However, the ENAT alone is not sufficient, and the focus should be placed on individualised patient education, which should be tailored according to their needs.74
One area where patient education is particularly key is in promoting the role of physical exercise in axSpA. Although the most effective exercise protocol in axSpA has not been clearly established, physical exercise is shown to improve disease activity and physical function in individuals with SpA. Despite this, physical activity is lower in adults with axSpA compared to the general population, with higher disease activity levels in those who are less physically active.77 Many individuals with axSpA lack motivation to partake in physical activity, even when aware of the benefits.78 Additionally, many individuals with rheumatic conditions are unaware of physical activity guidelines.79 A 3-month behavioural intervention in axSpA that incorporated motivational interviewing techniques resulted in better QoL, spinal mobility, and physical activity levels in patients with axSpA compared to a control group, which was sustained at Month 6.80 This highlights the positive role that patient education can play in the management of axSpA.
CONCLUSION
In summary, axSpA is a chronic inflammatory condition, with both spinal and extraspinal manifestations. Recognition of IBP can be challenging, but the most recent ASAS IBP criteria have increased the sensitivity and specificity of the classification criteria. HLA-B27 is thought to play a significant role in the pathophysiology, but more research is needed to fully elucidate the pathways. Awareness of extraspinal manifestations of axSpAis is low, as shown by the infrequent screening that occurs. Regularly assessing multiple different disease outcomes with validated tools is an important part of managing axSpA, and assessing the effect of treatment. Enhancing patient involvement in their own management is also key.







